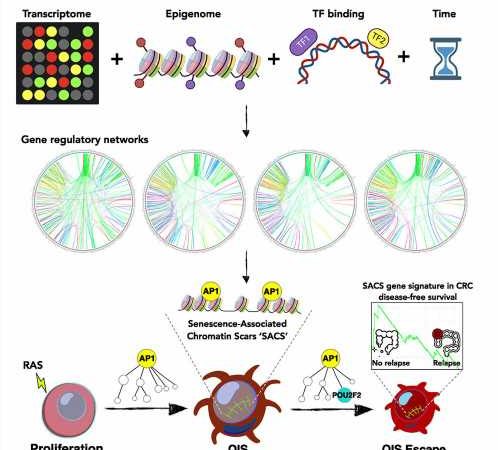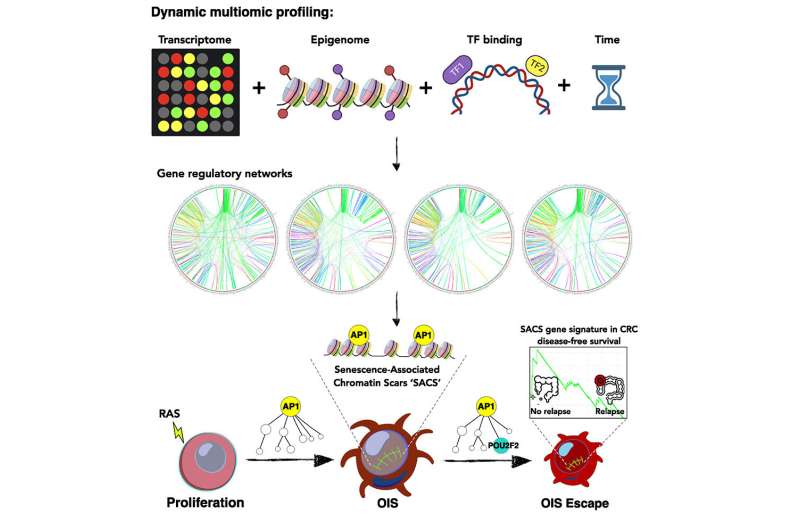
Mutating cells can prevent the spread of cancer by flipping themselves into a state of reduced activity called senescence. Cancer genes, however, can retaliate by reviving those cells so they can replicate again.
The mechanism for reviving senescent cells, sometimes called zombie cells, was only partially understood. Now, research from Rutgers has traced the process in colorectal cancer cells, and the investigators believe the process is similar in other tumor types.
“Once a cell starts to become cancerous, it begins replicating very quickly, and that triggers senescence,” said lead author Ricardo Iván Martínez-Zamudio, assistant professor of pharmacology at Robert Wood Johnson Medical School and research member of Rutgers Cancer Institute of New Jersey of the study published in Cell Genomics. “Once the cell becomes senescent, however, it often begins making a particular protein that helps it emerge from senescence.”
“The next stage in the research is to see if medications can target this protein,” Martínez-Zamudio said. “We want to find a substance that will bind to it and prevent it from binding with other proteins, so these cells stay in senescence and don’t reproduce.”
Researchers from the Herbig laboratory at Rutgers New Jersey Medical School began by examining the progression of cultured cells in Petri dishes. They then confirmed their findings in tissues taken from actual colon cancer patients.
The study revealed that entry into and exit from senescence is precoded and mediated by the very same types of proteins, AP1 transcription factors. These proteins not only spur entry into senescence but also promote escape from it by facilitating necessary protein interactions.
The study identified POU2F2 as a critical protein promoting escape from senescence and showed its role in colorectal cancer development. Overexpression and increased activity of POU2F2 are associated with cell inflammation and proliferation as well as decreased patient survival. POU2F2 has been implicated in the progression of various cancers and might be a viable drug target.
Any strategy to prevent escape from senescence might help, researchers said. A subset of tissue samples exhibited gene signatures that kept cells in senescence rather than allowing them to escape, and those patients were more likely to survive their cancers than patients whose cells escaped senescence.
Researchers traced the progression from cell senescence to escape with a technique called time-resolved multi-omics profiling, which allowed them to see which genes turned on and off and which proteins became more and less common over time.
Once this technique revealed that AP1 transcription factors were particularly active before cells escaped from senescence, the researchers turned off the genes that create such proteins and found that the cells could no longer spring back to life and begin reproducing.
“The body protects itself against some tumor types by having cells kill themselves entirely rather than downregulate into senescence,” said Martínez-Zamudio.
“We’re not entirely sure why that’s not a more common response—possibly because killing a large number of continuous cells would create holes in important tissues—but the body prefers senescence over cell death as a guard against many solid-tissue tumors, so we want to help that defense work properly.”
More information:
Ricardo Iván Martínez-Zamudio et al, Escape from oncogene-induced senescence is controlled by POU2F2 and memorized by chromatin scars, Cell Genomics (2023). DOI: 10.1016/j.xgen.2023.100293
Journal information:
Cell Genomics
Source: Read Full Article