In neurons, changes in the size of dendritic spines—small cellular protrusions involved in synaptic transmission—are thought to be a key mechanism underlying learning and memory. However, the specific way in which these structural changes occur remains unknown. In a study published in Cell Reports, researchers from Nara Institute of Science and Technology (NAIST) have revealed that the binding of cell adhesion molecules with actin, via an important linker protein in the structural backbone of synapses, is vital for this process of structural plasticity.
Actin proteins make up an important part of a cell’s structure, or cytoskeleton, and allow for dynamic changes in this structure by forming microfilaments when growth or movement is required. It was originally thought that the polymerization of actin was all that was needed for dendritic spines to change size in response to synaptic activation, but researchers at NAIST found that this process alone was not enough to cause structural plasticity, and decided to address this problem.
“Current models of structural plasticity in dendritic spines do not take mechanical force into account,” says Naoyuki Inagaki, corresponding author. “We had already identified the role of shootin1a, a protein involved in neuronal development, in axon growth and so we wanted to investigate whether this protein might also have a role in the structural plasticity of dendritic spines.”
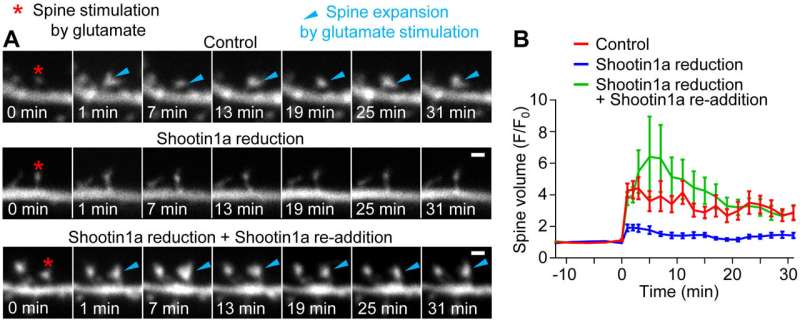
To explore this question, the researchers used neurons of control and shootin1a knockout rodents to examine whether shootin1a was involved in the formation of dendritic spines. The researchers wanted to determine if mechanical force was generated in dendritic spines by the shootin1a-mediated coupling of actin and cell adhesion molecules—cell-surface proteins that bind cells together at synapses—similar to what they had observed in axons.
“The results were clear,” explains Inagaki. “We found that shootin1a mechanically linked polymerizing actin with cell adhesion molecules in dendritic spines, and revealed that synaptic activity enhanced this coupling, thus allowing the actin filaments to push against the membranes and enlarge spines.” The results of this study are the first to link mechanical force with synaptic activity-dependent dendritic spine plasticity and provide new insights into the mechanisms of structural plasticity in these spines.
Source: Read Full Article
