.jpg) Thought LeadersAnnette BakHead of Advanced Drug Delivery in Pharmaceutical Sciences AstraZeneca
Thought LeadersAnnette BakHead of Advanced Drug Delivery in Pharmaceutical Sciences AstraZeneca In this interview, News-Medical Life Sciences talks to Annette Bak, Head of Advanced Drug Delivery in Pharmaceutical Sciences, R&D at AstraZeneca, about mRNA therapeutics and their analytical challenges in pharmaceutical development that she will present at Pittcon.
What are mRNA therapeutics and why are they important to medicine?
RNA-based therapeutics can be used to modulate disease-associated proteins. Protein overproduction can be druggable by small interfering RNA (siRNA) and antisense oligonucleotides (ASO). In this case, the RNA therapeutics inhibit the translation of the mRNA to the disease-causing protein.
Conversely, protein underproduction can be treated using messenger RNA (mRNA) to lead to translation of a desirable disease-modifying protein. RNA therapeutics are important to medicine as they are able to inhibit or produce proteins directly in the cell, which can be hard to do with other therapeutic modalities. The response is also transient.

A strand of RNA. Image Credit: nobeastsofierce / Shutterstock.com
Can you describe your research that you are presenting at Pittcon ‘mRNA Therapeutics – Analytical and Bioanalytical Characterization’?
mRNA therapeutics are not without challenges. The molecules are large, charged, and usually require a delivery system to elicit their actions in cells. The unique nature also creates analytical challenges in pharmaceutical drug discovery and early development.
My presentation at Pittcon will look at nucleotide-based therapeutics case studies, including the analytical and bioanalytical characterization in late discovery and early development.
How do you characterize mRNA therapeutics using analytics and bioanalytics?
The point of my presentation is that analytical characterization of RNA therapeutics is very different from other therapeutic molecules such as small molecules and proteins. In the discovery phase, there is a need to characterize the RNA molecule and the delivery system in cells in order to understand the mechanism of action and delivery system performance.
This produces new analytical and automation challenges as high-throughput systems need to be developed to design, make, test and analyze drug delivery systems. In early development, these new modalities give rise to distinct impurities and degradation products, which necessitates the development and validation of complex analytical methods for quality control (QC) purposes. These will typically be very different than other modalities which is both a scientific opportunity and a challenge.
From transport and turning genes on or off to playing part in chemical reactions and protein building, the versatility of RNAs has become a focus in medical research. What makes mRNA specifically useful for therapeutic research?
The ability to turn the production of disease-causing proteins off (i.e., with ASOs, siRNA) and turn the production of beneficial proteins on (i.e., with mRNA) means that RNA constructs are not only useful as therapeutics but also to study the biology and pharmacology of diseases. RNAs are therefore also useful as research tools.
mRNA therapeutics has been called “disruptive” to the drug industry. Why and what does this mean?
All mRNA is generated with four different building blocks, which when put together in a unique sequence order have the capacity to encode for all varieties of proteins. Once the industry gains familiarity with the therapeutic class, these characteristics have the potential to allow for rapid development of mRNA therapeutics and its drug development path which is a very exciting prospect.
Pittcon 2021: March 8-12
Register for Pittcon 2021
mRNA therapy essentially enables the body to make its own proteins for various functions. How are mRNA therapeutics shaping the ideas of personal and individualized therapy from our own bodies?
RNA is up and down-regulated in various disease states, for example, leading to the production of certain disease-causing proteins or underproduction of others. With RNA-based therapeutics, which falls in the class of precision medicine, one has the potential to specifically dial the production up or down within the patient’s own body leading to a therapeutic that is in essence the patient’s own rather than a foreign intervention. This may lead to better impact of the potential medicine.
Illustration of cancer cells. RNA dysregulation can have a part in cancer development. Image Credit: nobeastsofierce / Shutterstock.com
How would you describe the evolution of pharmaceutical technologies, including mRNA therapeutics, from the past to the present?
We have definitely seen changes in the modality and drug delivery spaces with several new therapeutic approaches such as RNA-based medicine making an entry recently. It is due to business changes in the industry but also changes in capabilities in the scientific community, and has led to a different approach to drug discovery.
Previously the target was identified, then the modality selected, and finally, the delivery strategy was determined in a serial fashion. The scope of drug delivery was relatively narrow. Now the scope has expanded, and once the target has been selected all modalities and delivery options are considered in parallel.
In other words, this approach allows for the section of the modality that is optimally suited for the target in question. The complexity of drug delivery has also increased and more modalities and routes are now in play along with intracellular and extended-release delivery options.
Therefore in the discovery phase, close collaboration between design and delivery scientists is a “must” in the emerging parallel and more complex approach. It is also important that we build in the clinical translational element into pre-clinical development so that new technologies do not stall as interesting preclinical concepts.
At AstraZeneca, the diversity of technologies applied in our early pipeline is exemplified by the increased number of new modalities entering clinical development. 30% of our early pipeline now consists of new drug modalities, including oligonucleotides and mRNA.
Could you describe some of the challenges you encountered while characterizing mRNA therapeutics?
Developing an R&D roadmap for a new therapeutic class such as mRNA is both exciting and challenging. My research focuses on Drug Delivery and Chemistry Manufacturing and Controls (CMC), in other words deriving the product or dosage form that may eventually reach the patient.
In this context for a new therapeutic modality, we need to develop new delivery systems, formulations, analytical methods, and frameworks for regulatory submissions in order to get a therapy approved. To give an example that I will also cover in my presentation, since mRNA therapeutics works in the cell, tracking of the delivery system in the cell becomes a necessity, and as a result, when setting up my department a few years back we had to build a cell biology group. This is a new concept in formulation departments in the industry.
What other applications for mRNA therapeutics, besides vaccines, do you expect or would like to see as research progresses?
Any disease that could benefit from the production of a certain protein is in theory druggable by an mRNA. The major benefit comes when the event occurs in the cell, as it is very hard to get protein replacement therapy into cells. A recent application is gene editing using the CRISPR/CAS system where the guide RNA and the CAS 9 protein can be delivered as an RNA.
The guide RNA will then position the CAS 9 enzyme on the DNA to correct faulty genes. We are seeing such therapeutics progress in pharmaceutical pipelines, especially for rare genetic diseases.
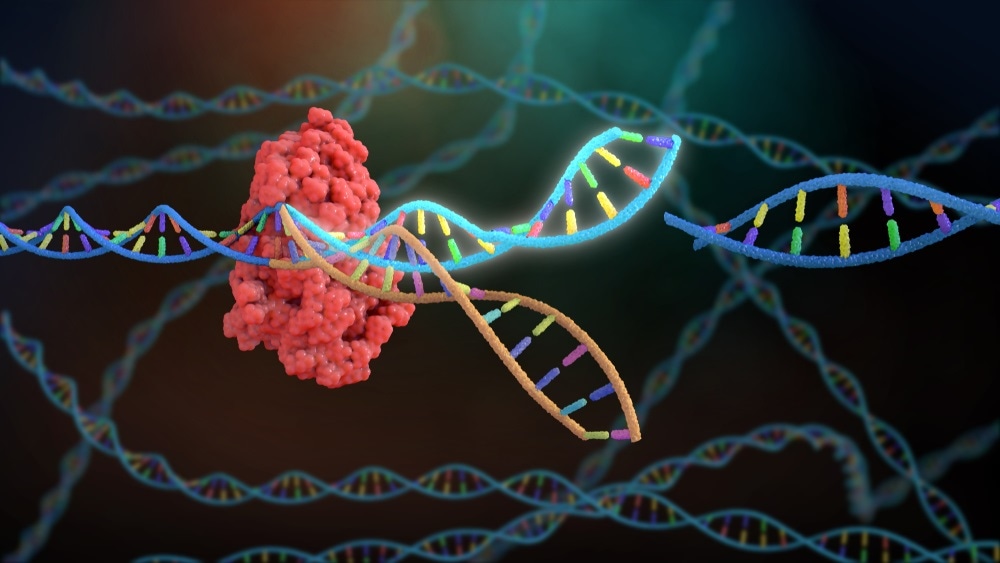
CRISPR/CAS gene editing. A recent application of RNA therapeutics is in gene editing using the CRISPR/CAS system, where the guide RNA and the CAS 9 protein can be delivered as an RNA. Image Credit: Nathan Devery / Shutterstock.com
What is in store for you and your research in mRNA therapeutics?
The therapeutic class is still relatively new and developing fast. We are currently investigating the potential of mRNA in a number of therapy areas with our partner Moderna and we are also advancing the use of novel lipid chemistries and lipid nanoparticles to promote the delivery of mRNA therapeutics.
Looking ahead, we need to be on our feet to:
- Derive new partnerships to internalize great delivery systems in addition to what we are developing internally
- Find ways to administer the class by different delivery routes of convenience for the end-user
- Continue to develop new CMC processes to progress a variety of RNA therapeutics in the pipeline
You have worked across different countries like the UK, US, Sweden, and China. How have you seen drug delivery and the therapeutic industry collaborate across borders?
Over the course of my career science has become global and collaborations outside company borders are now much more commonplace, and even expected. The resulting creativity increase is an impacting factor in the explosion of therapeutic modalities and drug delivery approaches.
We know the best science doesn't happen in isolation and to address all the issues facing healthcare and achieve the scientific breakthroughs we aspire to, we need to work together, collaborating among ourselves and with others, bringing the best talent to bear on the toughest problems.
In many ways, I think the pandemic has made global collaborations easier due to the increase in online meeting aptitude and culture.

The importance of global collaboration in the pharmaceutical industry has been highlighted during the COVID-19 pandemic. Image Credit: pogonici / Shutterstock.com
As the lead of AstraZeneca’s Advanced Drug Delivery Team, how has your work been impacted this year?
I relocated back to the US during summer 2020 and have large teams back in Sweden and the UK. Due to the pandemic, I have been unable to visit them. Since my team is in global locations I have always had many virtual meetings, but those have of course increased in frequency.
After a practice period, they are now quite seamless. Even post the pandemic I suspect that I will travel less. Separately I spoke about science creativity but in 2020 we also applied significant operational creativity to get work done under these different circumstances. It includes, for example, scheduling lab-based activities to allow for social distancing, conducting online rather than face-to-face interviews when recruiting, online lunches and coffee chats plus many more.
As President-Elect for the American Association of Pharmaceutical Scientists, what do you see for future roles of pharmaceutical scientists in medical and health industries?
Pharmaceutical scientists will have an important role to play in the future. The science landscape has changed with the pandemic of course but I also mean this in terms of more modalities, more difficult targets, and more collaborative efforts within and outside companies.
Therefore it is important that we continue to educate and develop ourselves to stay current and relevant for the new therapeutic approaches such as RNA-based medicines. Professional associations such as AAPS play important roles in advancing science, collaborating with other associations, expanding the global reach and career development for pharmaceutical scientists.
Due to the increase in collaborative efforts outside company borders, soft skills offerings by science professional organizations such as AAPS will be impactful to develop pharmaceutical scientists for future more outward-facing roles.
You are a large advocate for career development for women and chartered the “Women in Pharmaceutical Sciences” movement. Could you describe the mission and activities of this movement?
An AAPS collaborator, Nur Zaveri, and I started the effort back in 2013. We realized that we both wished had had more support when we were starting our careers. Therefore we coined the idea of a forum where women and other diverse groups could share experiences.
What made the effort particularly great is the interest and willingness of the women of AAPS to step up and share their experiences – be that struggles, victories, or career strategies. Now, after six symposia at AAPS meetings, several articles in the AAPS Newsmagazine by us and others just to name a few activities, we have a flourishing AAPS community in this area.
 Annette Bak is a strong advocate for women in pharmaceutical science. Image Credit: MicroOne / Shutterstock.com
Annette Bak is a strong advocate for women in pharmaceutical science. Image Credit: MicroOne / Shutterstock.com
What advice do you have for early-career women looking to start in pharmaceutical sciences?
My advice to young women hoping to embark on a career in the science field would be “follow your passion”. I considered medicine and veterinary medicine based on general perceptions of desirable careers but discovered quickly that pharmaceutical sciences were much better aligned with my skills, traits and interests.
Based on my experience of managing people across three large pharmaceutical companies, it is all too easy to go in the direction of what you “ought” to do, but success and therefore science impact comes from listening to yourself and finding what you “want to do” and what you are “passionate” about.
Pittcon 2021: March 8-12
Register for Pittcon 2021
Another piece of advice is to find a mentor or more than one. Not so much to grow scientifically, but to help you transition from studying and the educational system. No matter if you select industry, academia, or government employment it will require adjustment over graduate school or a postdoc. A mentor can also guide you early on to make the best career choices
What impacts do events like Pittcon have on the communication of your work?
The science community has become global and more collaborative, which has led to an increase in science diversity – in the context of my work, this is an increased number of therapeutic modalities and drug delivery systems.
That communication and exchange of science has an enhanced importance in today’s scientific community to enable the solving of increasingly complex problems. Presenting at conferences such as Pittcon is key in this context.
Why do you believe Pittcon is important in the therapeutic and science industry?
Pittcon is one of the largest analytical chemistry conferences and analytical chemistry is the backbone for quality in most if not all science industries.
Aligned with this mission it also very directly integrates instrumentation and instrument manufacturers in the technical program, which allow participants to leave with not only instrument brochures but also concrete examples of their application. Pittcon is a large conference and may have other purposes but this is why I personally look forward to attending.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.
