The ability to hear relies on neurons to constantly transmit information at very rapid timescales. This rapid rate of information transmission results in intense energetic demands. Within our cells, microscopic power plants called mitochondria provide the main source of energy keeping our bodies moving. While mitochondria serve an essential function throughout the body, within the brain they play an especially crucial role; providing the tremendous amount of energy needed to facilitate synaptic transmission (the transfer of information between neurons).
In the auditory system there is a large presynaptic terminal called calyx of Held that is critical for binaural sound processing. Prior to the onset of hearing, immature calyx synapses, do not release neurotransmitter at very fast rates. But once mature, they reliably and rapidly release neurotransmitter to encode auditory information., However, how mitochondria support the energy-demanding activity of the mature synapse remained unknown.
In a recent study published in the Journal of Neuroscience, scientists from the MPFI and the University of Iowa CCOM have provided unprecedented insight into the presynaptic distribution and profile of mitochondria in the developing and mature calyx of Held.
“My lab investigates how synapses enable neural circuits transmit a wide variety of information. In particular we are very interested in understanding the synaptic mechanisms that enable fast auditory signaling required for accurate identification and perception of sound information and their contribution to auditory deficits ,” explains Samuel Young Jr., Ph.D., former Research Group Leader at MPFI and now Associate Professor in the Department of Anatomy and Cell Biology at the University of Iowa CCOM. “While we understand some of the general principles of how the calyx enables proper sound processing , many are still unknown. Therefore, we wanted to understand if there were mitochondrial changes at the subsynaptic levels. To answer our question, we needed the expertise of the EM core at MPFI. What started as a fledgling idea and a simple conversation and, turned into a fruitful collaborative effort.”
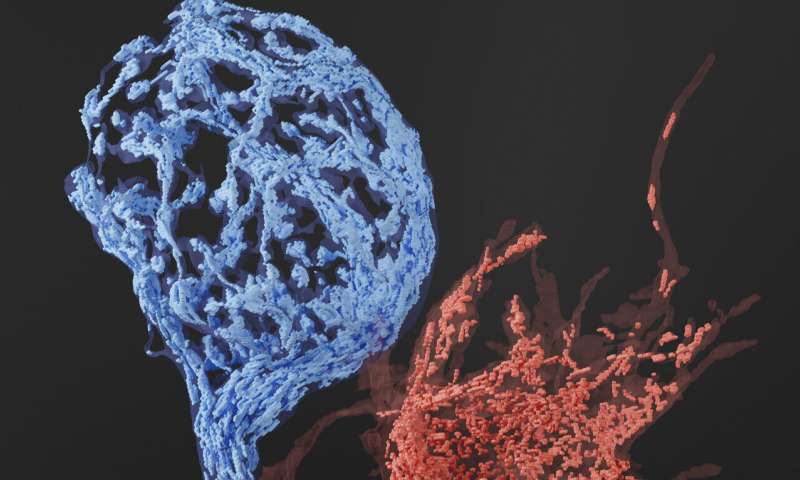
Due to their relatively small size, mitochondrial volume and distributions are often difficult to analyze using conventional methods and require 3-D- electron microscopy to fully reveal their intricate structural details. To accomplish this the Young Lab created a helper-dependent adenoviral vector with the mitochondria-targeting peroxidase, mito-APEX2 and expressed it at the mouse calyx of Held. In addition to that, the MPFI EM team developed protocols to detect APEX2-labeled mitochondria by 3-D- electron microscopy to carry out extensive analysis of the presynaptic mitochondria volume and abundance.
“Our biggest challenge was to develop protocols and workflows that would allow us to image mitochondria within the calyx in fine detail using the advanced 3-D- EM technologies,” describes Connon Thomas, EM assistant at MPFI and first author of the publication. “After extensive optimization, we devised two strategies; the first used serial block-face scanning electron microscopy or SBF-SEM for short, which is a type of specialized EM that allows us to generate a large-scale set of 3-D images in order to reconstruct and analyze mitochondria within the terminals. The second strategy used Automated Tape-collecting Ultra-Microtome serial section scanning electron microscopy (ATUM-ssSEM), which is a technique that produces higher resolution images which makes it easier to analyze fine sub-synaptic structures.”
3-D reconstructions of images taken with SBF-SEM revealed that mitochondrial volumes within the mature calyx and its surrounding axon were significantly higher than those found in its immature counterpart. It also seemed that mitochondria are selectively enriched within the mature calyx, containing higher volumes than the surrounding axon. This data affirms the idea that during development increased mitochondrial volumes support the higher energy demands of a more active mature calyx.
Using genetic tools developed by Young Lab and innovative new protocols developed by MPFI’s EM Core, their combined expertise has generated novel approaches with broad applications in neuroscience research. “The strong collaboration between our EM Core and the Young Lab was essential for the success of this work,” notes Naomi Kamasawa, Ph.D., Head of EM Core. Our collaborations will continue and will undoubted bring exciting new developments.”
Source: Read Full Article
