An international research team led by Nanyang Technological University, Singapore (NTU Singapore) has found that microorganisms living in the gut may alter the aging process, which could lead to the development of food-based treatment to slow it down.
All living organisms, including human beings, coexist with a myriad of microbial species living in and on them, and research conducted over the last 20 years has established their important role in nutrition, physiology, metabolism and behavior.
Using mice, the team led by Professor Sven Pettersson from the NTU Lee Kong Chian School of Medicine, transplanted gut microbes from old mice (24 months old) into young, germ-free mice (six weeks old). After eight weeks, the young mice had increased intestinal growth and production of neurons in the brain, known as neurogenesis.
The team showed that the increased neurogenesis was due to an enrichment of gut microbes that produce a specific short chain fatty acid, called butyrate.
Butyrate is produced through microbial fermentation of dietary fibers in the lower intestinal tract and stimulates production of a pro-longevity hormone called FGF21, which plays an important role in regulating the body’s energy and metabolism. As we age, butyrate production is reduced.
The researchers then showed that giving butyrate on its own to the young germ-free mice had the same adult neurogenesis effects.
The study was published in Science Translational Medicine yesterday (13 November), and was undertaken by researchers from Singapore, UK, and Australia.
“We’ve found that microbes collected from an old mouse have the capacity to support neural growth in a younger mouse,” said Professor Pettersson. “This is a surprising and very interesting observation, especially since we can mimic the neuro-stimulatory effect by using butyrate alone.”
“These results will lead us to explore whether butyrate might support repair and rebuilding in situations like stroke, spinal damage and to attenuate accelerated aging and cognitive decline.”
How gut microbes impact the digestive system
The team also explored the effects of gut microbe transplants from old to young mice on the functions of the digestive system.
With age, the viability of small intestinal cells is reduced, and this is associated with reduced mucus production that make intestinal cells more vulnerable to damage and cell death.
However, the addition of butyrate helps to better regulate the intestinal barrier function and reduce the risk of inflammation.
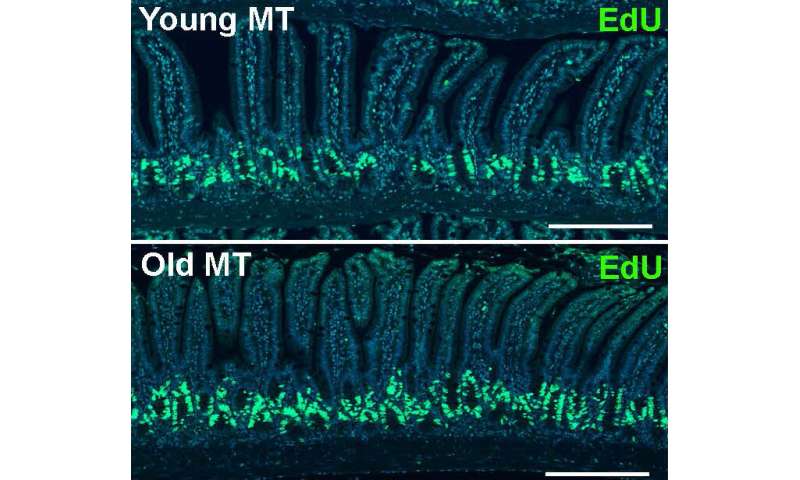
The team found that mice receiving microbes from the old donor gained increases in length and width of the intestinal villi—the wall of the small intestine. In addition, both the small intestine and colon were longer in the old mice than the young germ-free mice.
The discovery shows that gut microbes can compensate and support an aging body through positive stimulation.
This points to a new potential method for tackling the negative effects of aging by imitating the enrichment and activation of butyrate.
“We can conceive of future human studies where we would test the ability of food products with butyrate to support healthy aging and adult neurogenesis,” said Professor Pettersson.
“In Singapore, with its strong food culture, exploring the use of food to ‘heal’ ourselves, would be an intriguing next step, and the results could be important in Singapore’s quest to support healthy aging for their silver generation.”
Group leader Dr. Dario Riccardo Valenzano at the Max Planck Institute for Biology of aging in Germany, who was not involved in the study, said the discovery is a milestone in research on microbiome.
“These results are exciting and raise several new open questions for both biology of aging and microbiome research, including whether there is an active acquisition of butyrate producing microbes during mice life and whether extreme aging leads to a loss of this fundamental microbial community, which may be eventually responsible for dysbiosis and age-related dysfunctions,” he added.
Professor Brian Kennedy, director of the Centre for Healthy aging at the National University of Singapore, who provided an independent view, said, “It is intriguing that the microbiome of an aged animal can promote youthful phenotypes in a young recipient. This suggests that the microbiota with aging have been modified to compensate for the accumulating deficits of the host and leads to the question of whether the microbiome from a young animal would have greater or less effects on a young host. The findings move forward our understanding of the relationship between the microbiome and its host during aging and set the stage for the development of microbiome-related interventions to promote healthy longevity.”
Source: Read Full Article
