Cellular senescence, a state of permanent growth arrest, has emerged as a hallmark and fundamental driver of organismal aging. It is regulated by both genetic and epigenetic factors. Despite a few previously reported aging-associated genes, the identity and roles of additional genes involved in the regulation of human cellular aging remain to be elucidated. Yet, there is a lack of systematic investigation on the intervention of these genes to treat aging and aging-related diseases.
How many aging-promoting genes are there in the human genome? What are the molecular mechanisms by which these genes regulate aging? Can gene therapy alleviate individual aging? Recently, researchers from the Chinese Academy of Sciences have shed new light on the regulation of aging.
Recently, researchers from the Institute of Zoology of the Chinese Academy of Sciences (CAS), Peking University, and Beijing Institute of Genomics of CAS have collaborated to identify new human senescence-promoting genes by using a genome-wide CRISPR/Cas9 screening system and provide a new therapeutic approach for treating aging and aging-related pathologies.
In this study, the researchers conducted genome-wide CRISPR/Cas9-based screens in human premature aging stem cells and identified more than 100 candidate senescence-promoting genes. They further verified the effectiveness of inactivating each of the top 50 candidate genes in promoting cellular rejuvenation using targeted sgRNAs.
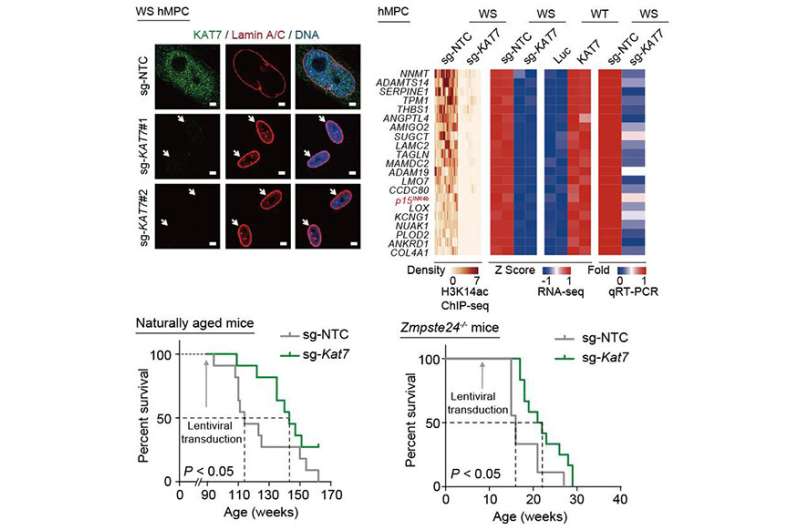
Among them, KAT7 encoding a histone acetyltransferase was identified as one of the top targets in alleviating cellular senescence. It increased in human mesenchymal precursor cells during physiological and pathological aging. KAT7 depletion attenuated cellular senescence, whereas KAT7 overexpression accelerated cellular senescence.
Mechanistically, inactivation of KAT7 decreased histone H3 lysine 14 acetylation, repressed p15INK4b transcription, and rejuvenated senescent human stem cells.
Cumulative studies have described that age-associated accumulation of senescent cells and proinflammatory cells in tissues and organs contribute to the development and progression of aging as well as aging-related disorders. Prophylactic ablation of senescent cells mitigates tissue degeneration and extends the healthspan in mice.
In this study, the researchers found that intravenous injection of a lentiviral vector encoding Cas9/sg-KAT7 reduced the proportions of senescent cells and proinflammatory cells in the liver, diminished circulatory senescence-associated secretory phenotype (SASP) factors in the serum, and extended healthspan and lifespan of aged mice.
These results suggest that gene therapy based on single-factor inactivation may be sufficient to extend mouse lifespan. The researchers also found that the treatment with the lentiviral vector encoding Cas9/sg-KAT7 or a KAT7 inhibitor WM-3835 alleviated human hepatocyte senescence and reduced the expression of SASP genes, suggesting the possibility of applying these interventions in clinical settings.
Altogether, this study has successfully expanded the list of human senescence-promoting genes using CRISPR/Cas9 genome-wide screen and conceptually demonstrated that gene therapy based on single-factor inactivation is able to delay individual aging. This study not only deepens our understanding of aging mechanism but also provides new potential targets for aging interventions.
Source: Read Full Article
