The rapid outbreak of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) virus has caused the ongoing coronavirus disease 2019 (COVID-19) pandemic. Vaccination programs have commenced in many countries around the world and scientists are conducting active research to understand the durability of immune responses post-vaccination.
A new study has been published on the medRxiv* preprint server that assesses antibody and functional T-cell responses at 6 months after complete vaccination with the Pfizer-BioNTech vaccine.
 Study: Evolution of SARS-CoV-2 immune responses in nursing home residents following full dose of the Comirnaty COVID-19 vaccine. Image Credit: Tequiero / Shutterstock.com
Study: Evolution of SARS-CoV-2 immune responses in nursing home residents following full dose of the Comirnaty COVID-19 vaccine. Image Credit: Tequiero / Shutterstock.com
About the study
Evidence gathered on messenger ribonucleic acid (mRNA) vaccines thus far shows that they are effective in reducing the incidence of symptomatic and asymptomatic SARS-CoV-2 infections and associated deaths.
These vaccines also have the desirable ability to elicit robust virus-specific T- and B-cell immune responses. However, the durability of antibody responses could be reduced in certain vulnerable sections of the population, such as the elderly and immunosuppressed.
In the current study, 46 nursing home residents were recruited, of which 44 were female and the median age was 89 years. Informed consent was obtained from participants, following which blood samples were collected in sodium heparin tubes 179-195 days after full vaccination.
Immunoglobulin G (IgG) and IgM antibodies against SARS-CoV-2 spike (S) and nucleocapsid (N) proteins were measured. The differences between medians were compared using the Mann–Whitney U-test or Wilcoxon test, where appropriate.
The Spearman rank test was used for correlation analyses between continuous variables. Two-sided exact P-values were reported and a P-value below 0.05 was considered statistically significant.
Study findings
In the study cohort of 46 individuals, 10 reported SARS-CoV-2 infection, as determined by a reverse-transcriptase polymerase chain reaction (RT-PCR) test and detection of N-specific antibodies. With respect to the SARS-CoV-2-S specific antibodies, data were available for 45 participants.
Overall, antibodies were found to decline by about 5-fold from the baseline to the follow-up period. Antibody waning was much more common among SARS-CoV-2 naïve residents as compared to those who had recovered from a previous SARS-CoV-2 infection.
All 46 individuals reported data on SARS-CoV-2-S specific T-cells. SARS-CoV-2-S interferon-γ (IFN-γ) T-cells were documented in 82.6% and 73.9% of residents at baseline and follow-up, respectively. The difference was highly significant (P=0.01). The corresponding figures for SARS-CoV-2-S IFN-γ CD8+ T-cells were 72% and 52.1%.
SARS-CoV-2-S IFN-γ CD8+ T-cell durability was found to be low. To this end, these cells were no longer detectable at follow-up in 16 out of 33 residents who had tested positive at baseline.
The situation was slightly different for SARS-CoV-2-S IFN-γ CD4+ T-cells, which were detected in 26% and 65.2% of residents at baseline and follow-up, respectively. Moreover, 19 participants developed CD4+ T-cell responses between the baseline and follow-up testing time points.
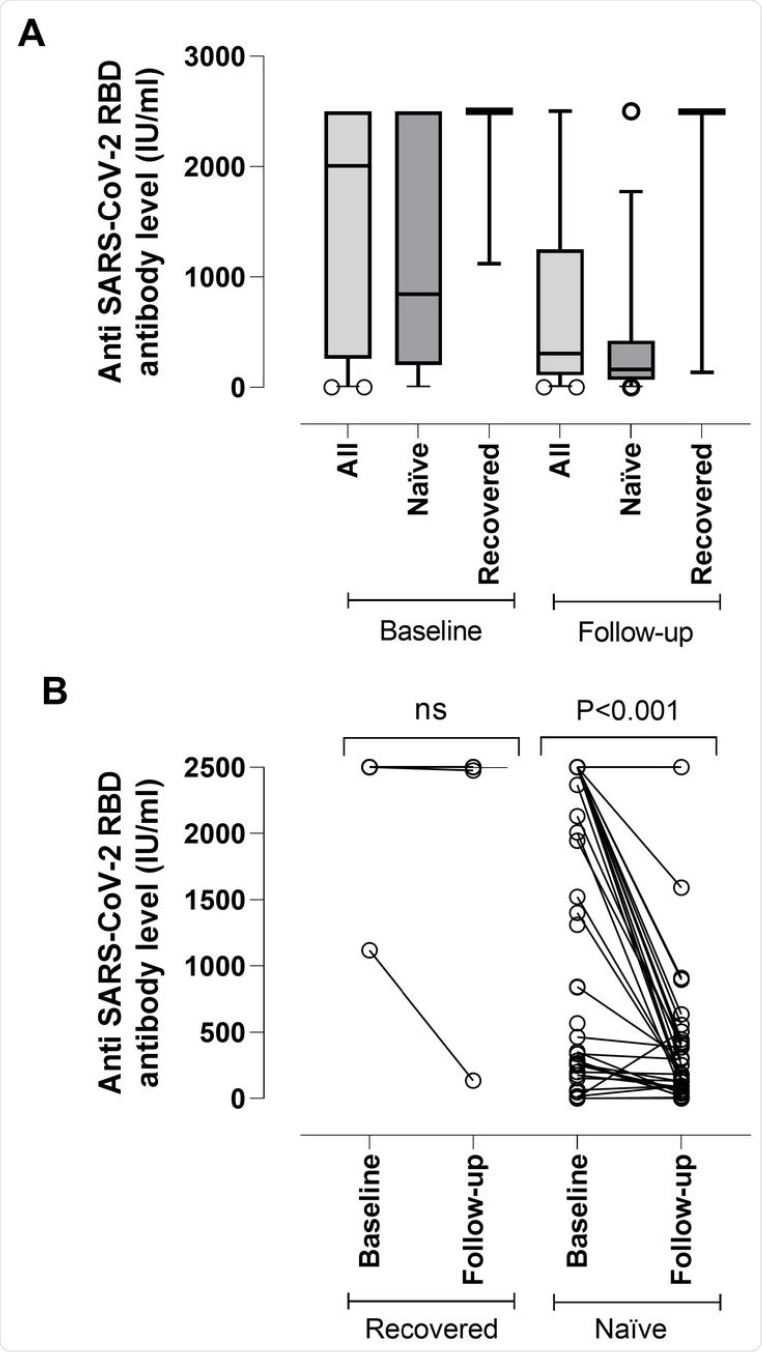 (A) SARS-CoV-2-S (RBD) plasma antibody (IgG and IgM) levels as measured by Roche Elecsys® Anti-SARS-CoV-2-S immunoassay in nursing home residents with (recovered) or without (naïve) documented prior SARS-CoV-2 infection at baseline (median, 17.5 days) and follow-up (median, 195 days) after complete Comirnaty® COVID-19 vaccination. The limit of detection of the assay is 0.4 IU/ml and its quantification range is between 0.8 and 250 IU/ml. Plasma specimens were further diluted (1/10) for antibody quantitation when appropriate. The assay is calibrated with the first WHO International Standard and Reference Panel for anti-SARS-CoV-2 antibody [12]. Bars represent median levels. (B) Individual kinetics of SARS-CoV-2-S (RBD) plasma antibodies in recovered and naïve nursing home residents. P-values for comparisons are shown (ns; not significant).
(A) SARS-CoV-2-S (RBD) plasma antibody (IgG and IgM) levels as measured by Roche Elecsys® Anti-SARS-CoV-2-S immunoassay in nursing home residents with (recovered) or without (naïve) documented prior SARS-CoV-2 infection at baseline (median, 17.5 days) and follow-up (median, 195 days) after complete Comirnaty® COVID-19 vaccination. The limit of detection of the assay is 0.4 IU/ml and its quantification range is between 0.8 and 250 IU/ml. Plasma specimens were further diluted (1/10) for antibody quantitation when appropriate. The assay is calibrated with the first WHO International Standard and Reference Panel for anti-SARS-CoV-2 antibody [12]. Bars represent median levels. (B) Individual kinetics of SARS-CoV-2-S (RBD) plasma antibodies in recovered and naïve nursing home residents. P-values for comparisons are shown (ns; not significant).
The results were also different across individuals who had recovered from SARS-CoV-2 infection and those who were naïve. For recovered individuals, the likelihood of having detectable SARS-CoV-2 IFN-γ CD8+ and CD4+ T-cells at follow-up was higher than in naïve individuals. SARS-CoV-2-S IFN-γ CD8+ T-cell frequencies decreased significantly over time, whereas the opposite was observed for CD4+ T-cells.
An interesting observation was that the residents who lacked anti-receptor-binding domain (RBD) antibodies at follow-up, exhibited SARS-CoV-2-S CD4+ T-cell responses. Scientists found no correlation between anti-RBD antibody levels and SARS-CoV-2-S IFN-γ CD4+ and CD8+ T-cells.
Conclusion
The main limitation of the current study is its small sample size, which makes inference difficult. This study also lacks a proper control group, as most of the controls included in previous studies were not available for follow-up sampling. Further, neutralization assays were not carried out.
Nevertheless, the current study documented that a substantial percentage of nursing home residents displayed SARS-CoV-2-S-reactive antibodies and T cell responses around 6 months after complete vaccination with the Pfizer-BioNTech COVID-19 vaccine. However, the antibodies were observed to decline over time.
The study also has implications for the booster (third) dose, which has been proposed for elderly people. To this end, the scientists indicate that the booster dose could be delayed beyond 6 months in fully vaccinated COVID-19 recovered residents.
*Important notice
medRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
- Gimenez, E., Alberola, J., Torres, I., et al. (2021) Evolution of SARS-CoV-2 immune responses in nursing home residents following full dose of the Comirnaty COVID-19 vaccine. medRxiv. doi:10.1101/2021.10.06.21264616. https://www.medrxiv.org/content/10.1101/2021.10.06.21264616v1
Posted in: Medical Research News | Disease/Infection News | Healthcare News
Tags: Antibodies, Antibody, Assay, Blood, CD4, Cell, Coronavirus, Coronavirus Disease COVID-19, Evolution, Heparin, Immunoassay, Immunoglobulin, Inherit, Interferon, Pandemic, Polymerase, Polymerase Chain Reaction, Receptor, Research, Respiratory, Ribonucleic Acid, SARS, SARS-CoV-2, Severe Acute Respiratory, Severe Acute Respiratory Syndrome, Syndrome, T-Cell, Vaccine, Virus

Written by
Dr. Priyom Bose
Priyom holds a Ph.D. in Plant Biology and Biotechnology from the University of Madras, India. She is an active researcher and an experienced science writer. Priyom has also co-authored several original research articles that have been published in reputed peer-reviewed journals. She is also an avid reader and an amateur photographer.
Source: Read Full Article
