A team of UF researchers has identified dozens of novel therapeutic targets for the development of antiviral therapies against COVID-19 and other coronaviruses that infect people.
A multidisciplinary team of researchers at the University of Florida is making progress in their search for targets that could be used for developing treatments for infections with COVID-19. The team recently posted a paper to bioRxiv reporting the identification of 53 novel genes and pathways that could become druggable targets for antiviral therapies for COVID-19 plus a broad array of coronavirus types.
Their work also identified existing compounds that target many of these same genes and pathways and that function as inhibitors to reduce viral growth. These antivirals are candidates for further testing as possible pan-coronavirus inhibitors, the team says, which could be effective at reducing the severity of infection across multiple types of coronaviruses.
The UF investigators used CRISPR gene editing techniques to search for genes and molecular pathways that aid in the replication of human coronaviruses. Team members include Stephanie Karst of the UF College of Medicine, Chris Vulpe of the College of Veterinary Medicine and Michael Norris of the College of Liberal Arts and Sciences.
Principal investigator Norris, a UF molecular biologist and bioengineer, says that identifying new or repurposed therapeutics is critical due to the increasing impact of new genetic variants in the pandemic.
“We need every possible weapon against this virus,” says Norris, who is a member of UF’s Emerging Pathogens Institute.
The importance of antiviral therapies
The discovery of broad-spectrum antiviral targets would be a useful tool in the fight against newly emerging genetic variants of SARS-CoV-2, the virus that causes COVID-19. While most of the world focuses on vaccine campaigns to end the COVID-19 pandemic, less attention has been paid to the parallel role of developing antiviral therapies.
Antivirals work by blocking or slowing a virus’s ability to replicate inside a host. These therapies typically target proteins produced by either the virus itself, or its host, and interfere with the pathogen’s ability to make more of itself.
Several factors underscore the need to develop antiviral therapies for COVID-19. First, future variants could potentially erode vaccine efficiency by evading detection by the immune system, which means it is important to keep searching for antiviral therapies that could help to lessen the severity of COVID-19 infections and symptoms.
Second, some people can’t receive vaccines, such as those with compromised immune systems, and a COVID-19 antiviral would provide a potent tool for mitigating the severity or length of infections.
Third, antivirals would help to relieve the burden of disease and slow transmission in regions or countries with little or no access to COVID-19 vaccines.
Screening cell cultures with CRISPR techniques
The research team used cultured cells that were experimentally infected with two different types of coronaviruses: SARS-CoV-2 and OC43. The first produces COVID-19 while the second produces a relatively mild and seasonally circulating common cold.
They included OC43 to zero in on targets that span both coronaviruses, which indicates that these could be broad-spectrum druggable targets, capable of being effective against a variety of coronaviruses known to infect humans. The appeal of a broad-spectrum antiviral target is that it could also prove effective against future coronaviruses that have not yet emerged but could be even more devastating than SARS-CoV-2.
The team used a gene-editing technique known as CRISPR to rapidly screen through all host genes to identify the few key genes that promote coronavirus infections in mammals. This approach helped the team pinpoint novel cellular pathways that the virus can use to replicate inside human cells. Inhibiting or silencing specific genes of interest allowed the team to confirm the role played by these pathways in the replication of the virus that causes COVID-19 and common colds.
“Identification of these pathways is key to designing new drugs or identifying existing inhibitors for the treatment or prevention of coronaviral infections,” Norris says. “Our work demonstrates that existing drugs can inhibit SARS-CoV-2 replication and the production of infectious particles in human lung cells.”
Genes and antiviral compounds of interest
The research team found that several host genes are important for both SARS-CoV-2 and OC43 infections, which means that these genes are considered candidates for broad-spectrum therapies against coronaviruses that infect humans.
Three genes—CDK4, EDC4 and XRN1—were found to play important roles in promoting replication pathways for the two coronaviruses. These genes could be important targets for developing antiviral therapies, the researchers say. If drugs could safely target them and inhibit their role, it would limit the coronavirus’s ability to replicate and spread within a host; in essence, controlling the infection and the symptoms it produces.
Three additional genes were identified as possibly supporting viral propagation from the host cell. GNPTAB, GNPTG and NAGPA were found to be involved in encoding for lysosomal proteins which the researchers speculate may promote the release of virions—the replicated progeny of a virus—from an infected cell.
Targeting these factors could lead to the development of a drug that prevents infected cells from releasing replicated virus. This effect might buy the immune system time to mount a more effective defense, while shortening the length and severity of an infection.
The study also identified 21 other genes that have been previously reported in other CRISPR screening studies, which validates the technique and the strength of the findings.
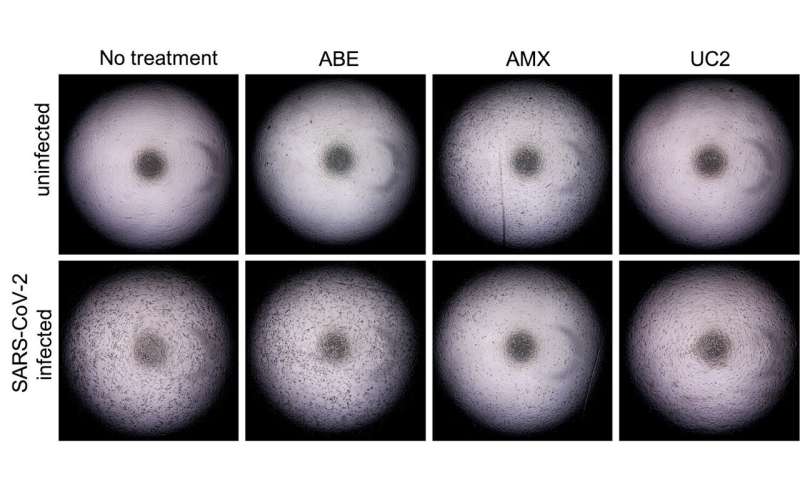
The team also screened multiple existing antiviral compounds which target the identified host genes to search for candidate anti-viral agents. Several compounds diminished coronavirus replication in cell culture experiments, including a cell cycle inhibitor, known as ABE, which targets CDK4.
“This compound and other candidate drugs which target the host capacity to support coronavirus replication hold great promise as a novel class of antiviral agents,” says principal investigator Vulpe. “Of course, additional work to validate these findings and demonstrate efficacy in more complex models, such as a mouse model, and ultimately in people, are necessary.”
Next steps
Norris says that the work to date has focused on virus-host interactions in cell cultures and that the team will next plans to test their findings in living organisms. With Karst as the principal investigator, the team received a 2021 UF Research Opportunity Seed Fund award to examine host RNA-processing dependencies of coronavirus infection first identified in the CRISPR experiments.
“Proving that these factors of interest prevent COVID-19 in an in vivo animal model is an essential step in the development of our potential therapeutics,” Norris says.
Vulpe notes that UF’s EPI is uniquely poised to support this work.
Source: Read Full Article
