In science, a simple but thorough observation can kick-start the most surprising findings. Researchers at the MUSC Hollings Cancer Center noticed that mice lacking a protein of interest in cancer research were showing visible signs of abnormal motor functions as they aged, including loss of coordination and strength.
The team showed that that lack of this protein resulted in the accumulation of damaged mitochondria that affected motor function. Treating these mice with a drug that triggered the destruction of damaged mitochondria restored their motor function. This research was reported in Aging Cell.
The symptoms of these mice were very similar to those seen in patients with amyotrophic lateral sclerosis (ALS). Commonly known as Lou Gehrig’s disease, ALS is a devastating neurodegenerative disease in which brain and muscle function decline over time. Few treatment options are available for ALS, and most patients die within two to five years of the diagnosis. The results seen after treating the study mice with the new drug show exciting promise for the treatment of ALS.
“We did not expect to detect any neurological damage in our mouse model that lacks our protein of interest, as they had no symptoms when they were younger. However, when they aged, we were able to see the signs of neurological problems, like ALS,” said Besim Ogretmen, Ph.D., SmartState Endowed Chair in Lipidomics and Drug Discovery at MUSC, who leads the team of researchers working on this project, which includes Natalia Oleinik, Ph.D., first author of the article, and Onder Albayram, Ph.D., who performed the behavioral studies establishing neurological deficits in the mice.
“Being observant and careful in the lab helped us uncover an interesting and unexpected mechanism that could result in innovative treatment options to overcome diseases like ALS in the clinic,” Ogretmen said.
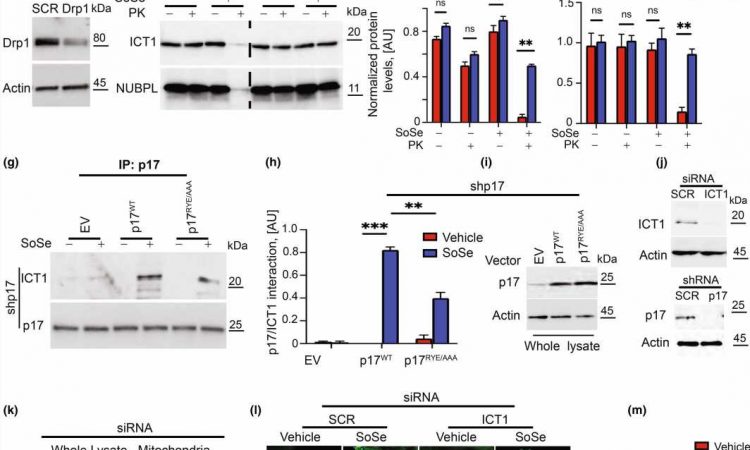
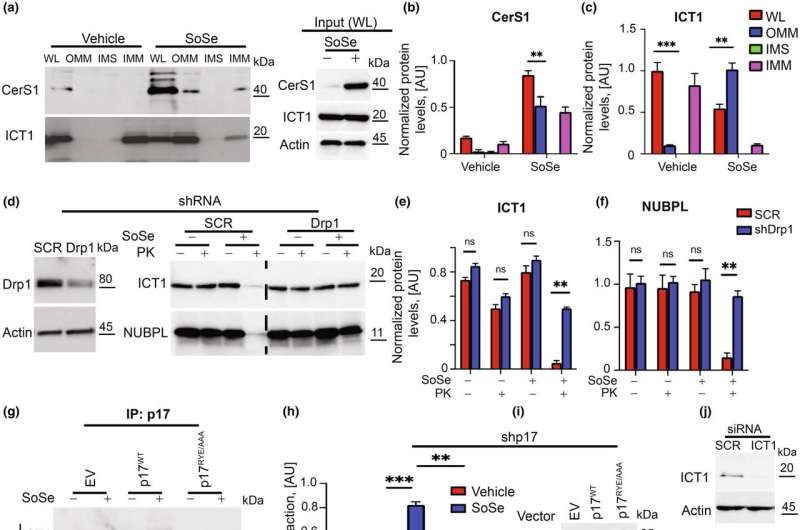
The mice in Ogretmen’s study lack a protein called P17/PERMIT, which his team has studied for years in the context of cancer. The protein moves a certain enzyme to the mitochondria, which are the powerhouses of the cell and needed for proper cell function. However, when mitochondria are damaged or malfunction, cells must get rid of them to remain healthy. This process by which they do so is called mitophagy.
Mitophagy is triggered by the actions of P17/PERMIT. The role of the enzyme it transports to the mitochondria is to make molecules called ceramides. The production of these ceramides in the mitochondria is what’s needed to start the destruction of mitochondria that aren’t working properly.
When P17/PERMIT doesn’t exist, as in the case of Ogretmen’s mice, then this whole process is disrupted. Damaged mitochondria will not begin to self-destruct, and they will accumulate.
Damaged mitochondria also accumulate in neurodegenerative diseases, like ALS, Parkinson’s and Alzheimer’s disease.
Once Ogretmen’s team understood the mechanism behind this accumulation, they realized they had already developed a drug in their cancer research that could target it.
“We have a drug that can get past this lack of P17 and restore this mitophagy process,” he said.
For cancer, the drug is given in high doses so that it will accumulate in mitochondria and cut off the power supply to tumor cells. For neurodegenerative disease, the drug is given in lower doses and is intended to keep cells healthy by preventing the accumulation of damaged mitochondria.
In the study, older mice treated with this drug regained some of their coordination and strength.
“They became almost like healthy mice of the same age,” Ogretmen explained.
While this drug is promising in mice, many challenges must be overcome before it will be useful in humans. One of the challenges that Ogretmen’s team had to work around was the fact that the drug was not able to pass through the barrier that protects the brain. They had to use special technology to allow the medication to get to the mouse brain with daily low doses.
In upcoming research, the Ogretmen team will explore new ways for this drug to pass through the barrier more efficiently. They will also explore treating other diseases like Alzheimer’s disease.
“I am hopeful that we can overcome these challenges so that the drug can be a potential therapeutic agent to treat people with these diseases,” said Ogretmen.
More information:
Natalia Oleinik et al, Alterations of lipid‐mediated mitophagy result in aging‐dependent sensorimotor defects, Aging Cell (2023). DOI: 10.1111/acel.13954
Journal information:
Aging Cell
Source: Read Full Article