Investigators have identified how the immune system can regulate organ rejection in mice, findings which may prove useful for improving transplant tolerance in humans, according to a recent study published in the Journal of Clinical Investigation.
More than 42,000 organ transplants were performed in 2022 according to OrganDonor.gov, but roughly 10% to 15% of transplant recipients will experience symptoms of organ rejection. Even if organs are initially accepted, secondary infections can induce inflammation, triggering rejection later on, said Zheng Zhang, MD, research professor of Surgery in the Division of Organ Transplantation and a co-author of the study.
“The reason that there is a rejection when we transplant organs from one individual to the other is that our cells have antigens and our immune system will recognize if the antigens are from ourselves or others. For foreign antigens, especially for transplant antigens, there are two major types: MHC class I and MHC class II, the two major histocompatibility complex antigens expressed in donor organs,” said Zhang, who is also a member of the Robert H. Lurie Comprehensive Cancer Center of Northwestern University as well as the Simpson Querrey Institute for BioNanotechnology.
“This paper is a mechanistic study looking at why and how these antigens, when activated by infection, can break down tolerance of a previously accepted graft,” said Zhang.
The study was a collaborative effort between Zhang and University of Chicago investigators Maria-Luisa Alegre, MD, Ph.D., professor of Medicine, and Anita Chong, Ph.D., Professor of Surgery.
In the study, investigators at the University of Chicago first analyzed how T-cells, the soldier cells of the immune system, reacted following a heart transplant in mice untreated or treated with an immunomodulating regimen, which consisted of donor splenic cells followed by a T-cell targeted protein, anti-CD154, known to induce transplant acceptance.
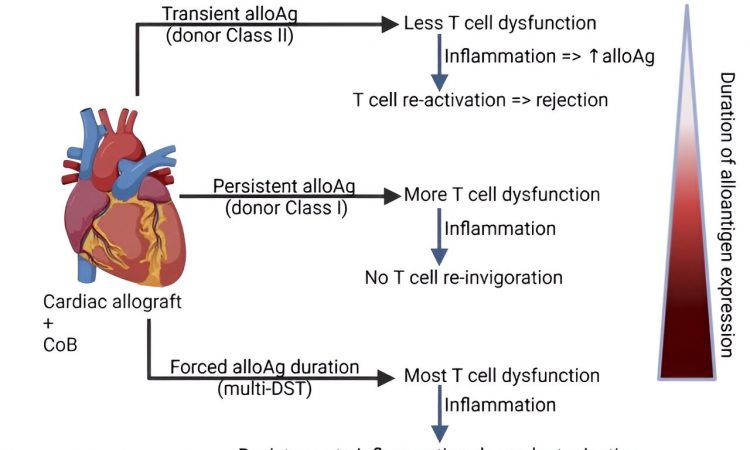
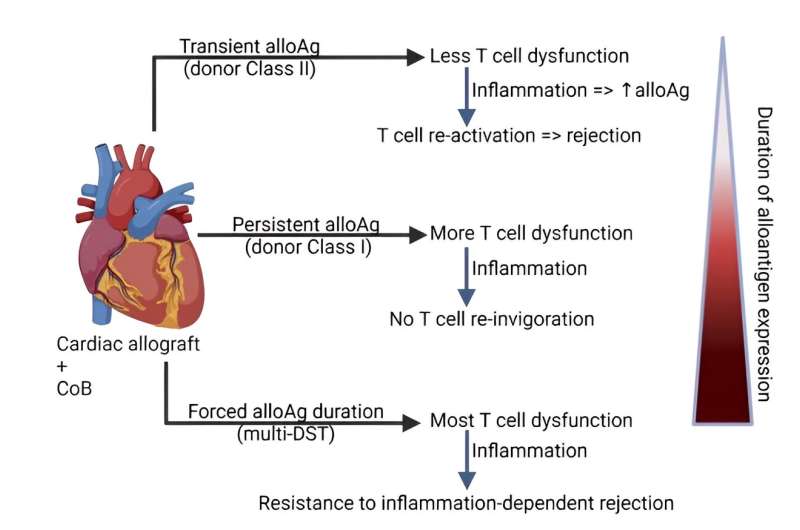
Using two different T-cell “tracers” that recognize antigens in the graft, they found that in transplant recipient mice who tolerated their donor grafts, TCR75 cells that recognize a donor MHC class I antigen exhibited signs of exhaustion, while TEa cells that recognize a donor MHC class II antigen remained functional.
Next, scientists infected mice at 30 days post-transplantation with listeria, which is known to lead to organ rejection. At eightdays post-infection, investigators analyzed the endothelial cells of the donor organ and found that those from infected mice had upregulated MHC class II levels compared to uninfected tolerant mice, resulting in activation of TEa cells and rejection.
Investigators hypothesized that exposing the recipient to all donor antigens over a long period of time after transplantation, including to the antigens that are otherwise transient in the graft, could desensitize the host’s TEa cells, lessening the likelihood of rejection.
To test this, investigators treated mice with anti-CD154 and administered either a single dose of donor spleen cells or administered several over the course of a month. They then analyzed the TEa cells of the mice and found that only the repeated injections induced exhaustion in the TEa cells.
At 35 days after heart transplantation, the graft-tolerant mice were infected with listeria. Mice that had been given multiple donor spleen cell injections showed fewer signs of rejection and had less inflammation and tissue damage than mice only given one, according to the study.
The findings suggest that priming T-cells of transplant recipients with repeated injections of antigens from their organ donor may help the recipient’s body avoid organ rejection as a result of infection. The results of the study also identify donor MHC class II as a problematic antigen that may be a cause of rejection after severe infections, said Zhang.
“When we infected transplanted mice with listeria, we found that the expression of donor MHC class II increased dramatically in the graft, a consequence of the cytokine release and inflammation caused by the infection, with several detrimental effects contributing to a rejection response,” Zhang said. “But repeated infusions of donor antigen, under cover of anti-CD154, can actually enhance tolerance. And this paper suggests that the duration of expression of the different donor antigens in the graft can influence how stable that tolerance is.”
Moving forward, Zhang and her collaborators will continue to study the mechanisms underlying transplant tolerance and the inflammatory events that make tolerance vulnerable, she said.
“By understanding this mechanism, we hope that we can actually find a way to make grafts more resistant to the detrimental effects of infections,” Zhang said. “These results will help clinicians design therapeutic interventions that can help sustain long-term tolerance.”
More information:
Christine M. McIntosh et al, Heterogeneity in allospecific T cell function in transplant tolerant hosts determines susceptibility to rejection following infection, Journal of Clinical Investigation (2023). DOI: 10.1172/JCI168465
Journal information:
Journal of Clinical Investigation
Source: Read Full Article