Gamma delta T cells, a special type of cell in the immune system, are incredibly effective at recognizing and killing cancer cells. Cancer patients with higher levels of these T cells in their tumors tend to fare better than those with lower levels. But scientists have struggled to understand exactly how gamma delta T cells can recognize cancerous cells, and how new cancer therapies may be able to take advantage of these powerful immune cells.
Now, researchers at Gladstone Institutes and UC San Francisco have pinpointed conditions that enable gamma delta T cells to identify cancer cells. The work was published in the journal Nature.
“We used the power of CRISPR genome editing to get fundamental insights into what can make cancer cells recognizable to gamma delta T cells so they can be targeted for elimination,” says Alex Marson, MD, Ph.D., director of the Gladstone-UCSF Institute of Genomic Immunology and senior author of the new study. “Our work opens the door to thinking about how this pathway could eventually be targeted in future immunotherapies.”
“This study gives us crucial insight into factors operating inside cancer cells that can trigger recognition and destruction by gamma delta T cells, one of the more potent assassins of the immune system,” adds Erin J. Adams, Ph.D., professor of biochemistry and molecular biophysics at the University of Chicago, who collaborated with Marson on this study.
An understudied cell type
T cells are a type of white blood cell responsible for recognizing trouble in the body—from invading viruses or bacteria to cancer cells with genetic mutations. There are billions of T cells patrolling your body, and they have a protein on their surface called a T cell receptor, which recognizes molecules on the target cells. Many experimental cancer treatments being studied today try to re-engineer T cell receptors so that T cells can better target tumors.
Previous studies have shown that a subset of T cells, known as gamma delta T cells, can be especially good at spotting cancer cells across various sites in the body. But at a molecular level, the conditions needed for gamma delta T cells to identify and clear cancer cells were unclear, making it difficult for scientists to envision ways to boost this process.
“We knew that gamma delta T cells recognize their target cells in a very different way from conventional T cells, but the field has had some trouble figuring out exactly how the gamma delta T cells were recognizing the cancer cells,” says Murad Mamedov, Ph.D., postdoctoral scholar at Gladstone and first author of the study.
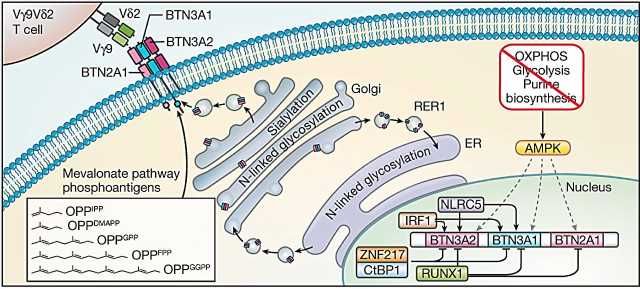
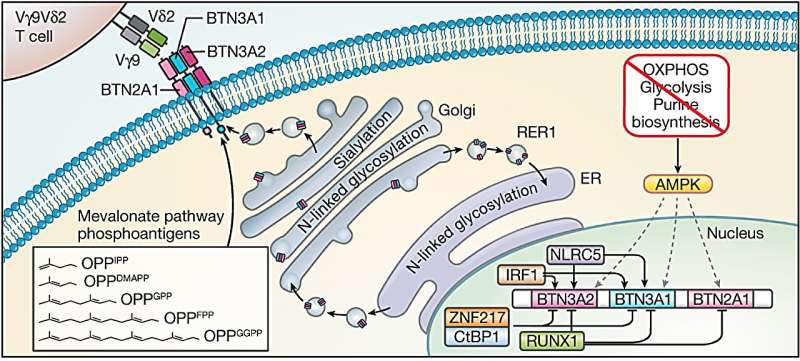
Mamedov, Marson, and their collaborators used CRISPR technology to disrupt thousands of genes in lymphoma cells and systematically test which gene disruptions affect whether or not the gamma delta T cells are killing cancer cells. They confirmed that the gamma delta T cells were recognizing a complex of molecules called butyrophilins, which were previously shown to be targeted by gamma delta T cells. But these molecules are found on the surface of many human cells—both healthy and diseased.
“In this case, it turned out that just knowing what gamma delta T cells recognize was not sufficient,” says Mamedov. “We needed to know how the butyrophilins are regulated and what makes them different in some cancer cells.”
To that end, the team confirmed another previously shown fact, that hyperactive cholesterol production—a common feature of many rapidly dividing cancer cells—led to activation of the butyrophilin complex, making it accessible to the gamma delta T cells.
A signal for stress
When they looked more closely at the results from their CRISPR screen, the researchers found that gene deletions that caused cellular stress and depleted energy production in cancer cells made these cells more likely to be killed by gamma delta T cells, and also increased the amount of butyrophilin molecules at the surface of cancer cells.
Using this insight, they showed that when tumor cells from cancer patients are treated with a drug that mimics a cell’s stress response, these tumor cells are more easily recognized by gamma delta T cells because of their increased butyrophilins, and as a result, are killed more efficiently.
“In healthy cells, butyrophilin is invisible to gamma delta T cells, so that T cells don’t start killing them,” explains Mamedov. “But when stress pathways are increased in cancer and the butyrophilins are activated, these molecules become more abundant and act as a target for gamma delta T cells.”
While the new results primarily shed light on the basic biology of how gamma delta T cells work and how they may have evolved, they also suggest that therapies manipulating the abundance of butyrophilin on the surface of patients’ cancer cells could make gamma delta T cells more effective cancer fighters.
“Much work lies ahead to design drugs that can boost cancer clearance by gamma delta T cells, but the findings of this team led by Murad move us a step closer by giving us fundamental insights into how gamma delta T cells recognize cancer targets,” says Marson.
“This amazing collaboration, which connected basic and clinical translational researchers, will allow scientists and physicians to not only better select patients for immune therapies using g9d2T cell receptors, but also to design novel compounds to enhance activity of these receptors,” says Jürgen Kuball, MD, Ph.D., one of the authors of the study, as well as professor of hematology and chair of the Department of Hematology at University Medical Center Utrecht.
More information:
Murad R. Mamedov et al, CRISPR screens decode cancer cell pathways that trigger γδ T cell detection, Nature (2023). DOI: 10.1038/s41586-023-06482-x
Journal information:
Nature
Source: Read Full Article