
Federal officials on Wednesday banned electrical shock devices used to discourage aggressive, self-harming behavior in patients with mental disabilities.
The announcement from the Food and Drug Administration follows years of pressure from patient groups and mental health experts who have called the treatment outdated, ineffective and unethical. The agency first announced its intent to ban the devices in 2016.
For years, the shock devices have been used by only one place in the U.S., the Judge Rotenberg Educational Center of Canton, Massachusetts, a residential school for people with autism and other psychiatric, developmental or mental disabilities.
School administrators have called the shocks a last resort to prevent dangerous behaviors, such as head-banging, throwing furniture or attacking teachers or classmates.
The FDA said Wednesday it estimates 45 to 50 people at the school are currently being treated with the device.
A woman who answered the phone at the center Wednesday morning said she could not comment on the FDA action.
Electric shocks and other painful or unpleasant treatments known as “aversive conditioning” were more widely accepted decades ago. But mainstream psychiatry now relies on behavioral modification, prescription drugs and other therapies that have proven more effective.
“Through advancements in medical science, there are now more treatment options available to reduce or stop self-injurious or aggressive behavior,” said Dr. William Maisel, a director in the FDA’s device center, in a statement.
The Rotenberg school has used shock devices carried in students’ backpacks, which were attached to their arms and legs via electrodes. School staffers could trigger a two-second shock to a patient’s skin by using a remote controller.
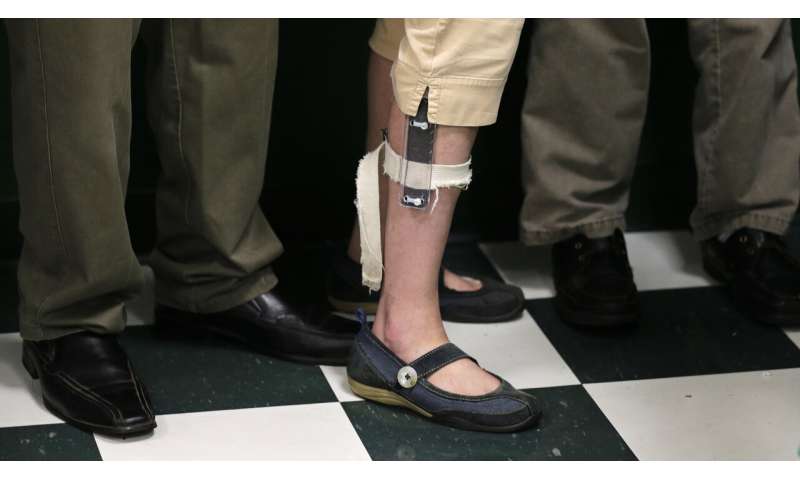
Some patients from the Rotenberg center have compared the shocks to a bee sting or worse. The school has faced several lawsuits brought by families who said their children were traumatized by the shocks.
Other parents reported that the technique was the only thing that prevented violent behavior linked to autism and other disabilities.
The FDA, echoing psychiatric experts, said in a statement that the therapy can exacerbate dangerous behaviors and lead to depression, anxiety and post-traumatic stress disorder. Patients have also suffered burns and tissue damage due to the device, the agency said.
Regulators said patients should instead receive treatments that focus on eliminating factors that trigger the behaviors or teaching patients coping skills to deal with them.
The FDA has only banned two other products in more than 40 years of regulating medical devices—powdered surgical gloves, which can cause allergic reactions, and fake hair implants, which caused infections and didn’t work.
Source: Read Full Article
