By adapting computer models originally developed to understand the biology of cancer cells, UCL scientists have identified new drug combinations with the potential to treat severe cases of COVID-19 infection at different stages of the disease.
Researchers say the findings could help lower the number of COVID-19 related deaths and reduce the strain on healthcare systems.
Published in npj Digital Medicine, the study tested the potential impact of interfering with different aspects of SARS-CoV-2 infection and the body’s responses to the virus. Results have identified existing therapeutics that might be suitable for treating COVID-19 patients.
Although vaccines and treatments for COVID-19 now exist, additional effective and affordable treatments are still urgently required. Cases of SARS-CoV-2 infection are still highly likely to occur, particularly when new variants arise.
Tackling virus replication and immune response
Therapeutic development for COVID-19 is complicated by the need to consider different stages of disease. Early symptoms are typically triggered by viral replication, while later and more severe disease is caused by the over-reaction of the body’s own immune defenses.
Different stages of disease are therefore likely to needed different treatments—and getting the timing wrong could have grave consequences: boosting immune responses in order to prevent viral replication could be highly damaging if they are already being ramped up.
The interplay between the virus, the cell it is infecting, and host immune responses involves a highly complex web of interactions. Interfering with these interactions using therapeutics could therefore have an effect throughout this web, which might help to clear the virus but could also disrupt important cellular processes and cause harmful side effects.
Model solutions
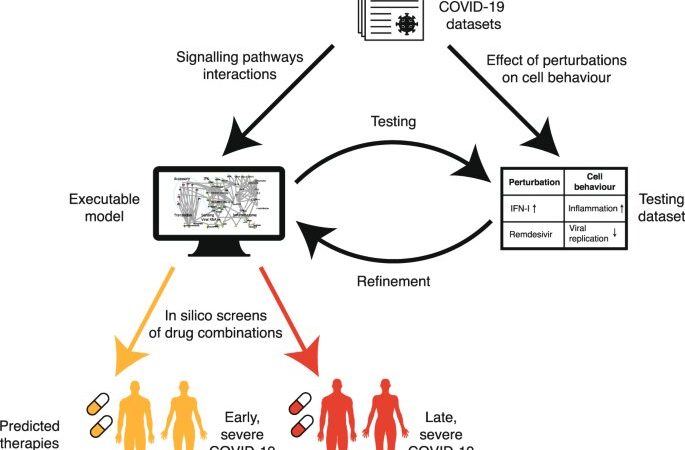
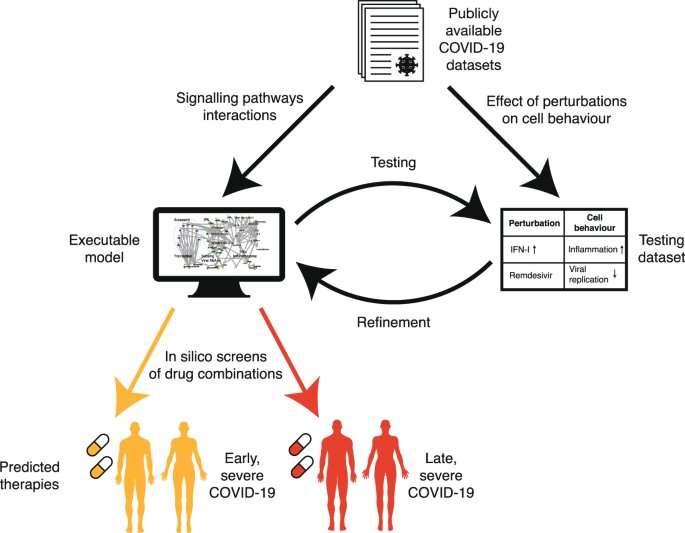
Similar issues have been faced by cancer researchers. To address this challenge, UCL researcher Professor Jasmin Fisher has developed computer models of cancer cell biology, which simulate the biochemical and metabolic pathways of cells and how they are subverted by cancer-causing mutations that drive uncontrolled growth of cells.
Using these models, the Fisher Lab can explore what might happen if particular pathways or cellular processes are inhibited, individually or in combination, so that the best targets for intervention can be identified and possible harmful side effects anticipated.
“We realized we could model SARS-CoV-2 infection using a computational framework originally developed in my lab to predict personalized treatment combinations for cancer patients and use it to predict effective repurposed drug combinations for treating COVID-19,” says Professor Fisher (UCL Cancer Institute), who was lead author of the study.
“We collated information available at the time on SARS-CoV-2 infection of airway cells and the immune response to infection, to create a dynamic model of viral infection and COVID-19 disease processes. Our study focused on two key stages—viral replication following initial infection (before severe symptoms emerge) and late-stage immune-driven disease, which is typically more severe,” says Professor Fisher.
The research team identified a range of therapeutic drugs, already licensed or in late development, that target processes thought to be important at these two stages. They then used their computer model to explore what might happen in cells when these processes were inhibited, mimicking the action of therapeutics. The model provided insights into impacts on virus replication and host responses, and the likely net effect in relation to both treatment of disease and safety.
Importantly, Professor Fisher and colleagues were able to validate their model by showing that the predicted effects of therapeutics already being used to treat COVID-19 at different stages of disease—such as antivirals and anti-inflammatory drugs—matched those seen in clinical studies.
In silico screening
Using this approach, the team examined 9,870 pairs of compounds acting on 140 potential cellular targets. They were able to identify new combinations of therapeutics that would be predicted to be beneficial at either early or late stages of disease, as well as the ‘windows’ when they might be safely deployed. For example, the combination of two drugs, Camostat and Apilimod, was predicted to have a particularly big impact on virus replication. This strong antiviral effect was confirmed using live SARS-CoV-2 cell culture assays by Dr. Ann-Kathrin Reuschl and Professor Clare Jolly in the Division of Infection and Immunity at UCL.
Source: Read Full Article