Scientists have discovered a new type of cell, SWAT, which can transform into fat cells depending on the microenvironment. A better understanding of the processes that balance the cell types could inspire innovative approaches to addressing cardiometabolic diseases.
Fat stored on the body—adipose tissue—contains many different cell types, including white fat (WAT) cells, which store lipids, and brown fat (BAT) cells, which burn lipids to produce heat and energy. Now, scientists at the University of Copenhagen and Rigshospitalet have discovered a new type of cell in the fat called SWAT, which gives adipose tissue its structural integrity.
In an article published in Nature Metabolism, the scientists explain how the discovery of SWAT cells could reshape our knowledge of how adipose tissues function and their crucial role in developing cardiometabolic diseases.
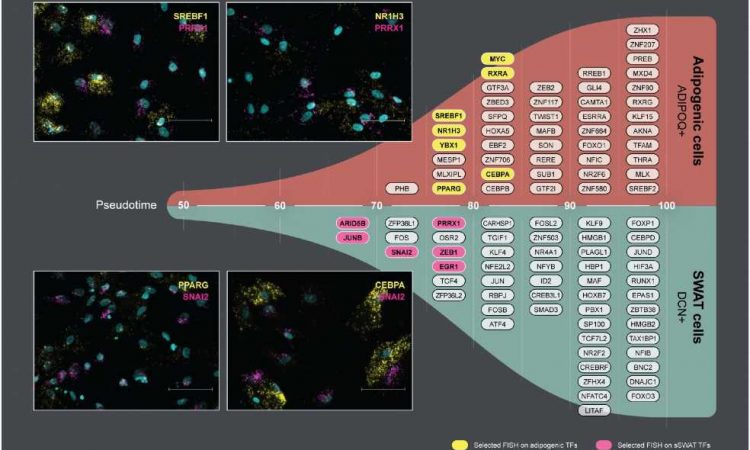
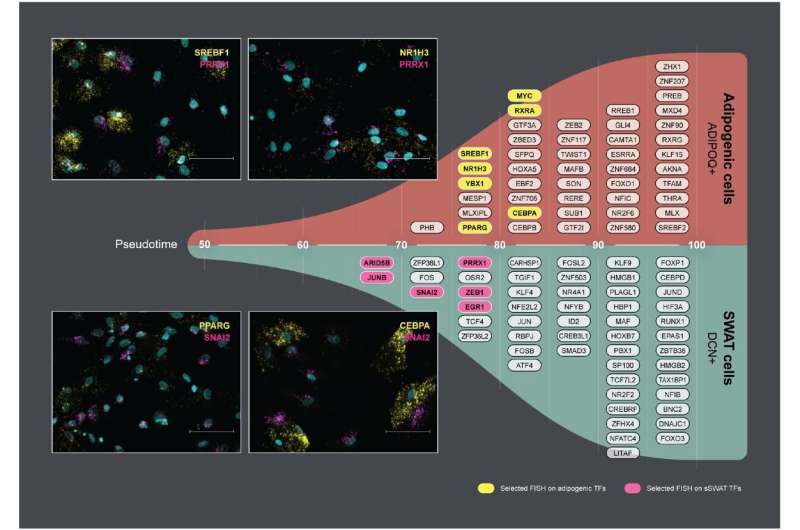
Structural Wnt-regulated adipose tissue-resident cells, or SWAT cells for short, release proteins that help to give adipose tissue its structure. However, the researchers were surprised to discover that SWAT cells develop from the same mother cells (progenitors) as fat cells. They also found that SWAT cells are flexible and can switch into several different cell types, including fat cells, or even back into progenitor cells.
“SWAT cells have the remarkable ability to revert to a progenitor-like state or differentiate into new adipogenic cells, depending on the microenvironment,” says Associate Professor Camilla Schéele from the Novo Nordisk Foundation Center for Basic Metabolic Research at the University of Copenhagen, and one of the co-senior authors of the paper.
Environment could dictate the cell’s fate
The flexibility of SWAT cells to revert into progenitor cells or further develop into WAT cells or BAT cells suggest that SWAT cells play an essential role in allowing adipose tissue to adapt to different metabolic conditions.
“We hypothesize that intercellular crosstalk maintains a delicate balance between the different cell types. This balance may be disrupted in an obesogenic microenvironment, potentially contributing to the development of cardiometabolic diseases,” explained senior researcher Søren Nielsen from Rigshospitalet, another co-senior author of the paper.
The scientists have yet to fully understand what signals control the development of the different cell types. However, they are optimistic that the study could inspire innovative approaches to address obesity-related health challenges by unraveling the intricate mechanisms that govern how adipocytes differentiate.
Researchers at the University of Massachusetts, U.S., also published a study in Nature Metabolism on the discovery of SWAT, with complementary findings.
More information:
Nagendra P. Palani et al, Adipogenic and SWAT cells separate from a common progenitor in human brown and white adipose depots, Nature Metabolism (2023). DOI: 10.1038/s42255-023-00820-z
Guan Wang et al, Maintenance of adipose progenitors in adipogenesis, Nature Metabolism (2023). DOI: 10.1038/s42255-023-00810-1
Journal information:
Nature Metabolism
Source: Read Full Article