Give credit to your dad’s gene for keeping you safe during those long months in your mother’s womb.
Without this genetic warrior, you might have succumbed to any number of viral infections that otherwise could be fatal to a fetus. A new paper published this week in the journal Cell Host & Microbe explains the mechanisms behind this anti-viral protection.
“What’s unique about this gene is how it produces a form of defense for the baby in the womb,” said Hana Totary-Jain, Ph.D., associate professor of Molecular Pharmacology and Physiology and Heart Institute at the USF Health Morsani College of Medicine and senior author of the paper.
Their research focused on viruses that affect a pregnant mother and consequently her fetus, which are highly vulnerable to infection because their immune systems are immature. Some viruses, including Zika, rubella, and other serious infections, are rarely transmitted from mother to fetus in utero and can cause devastating consequences.
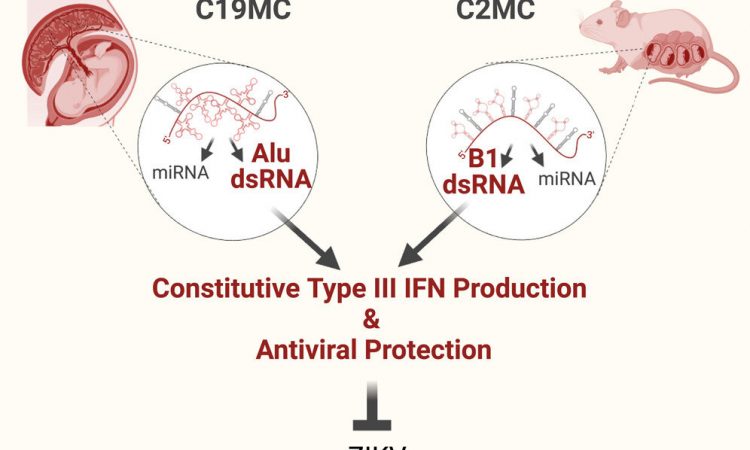
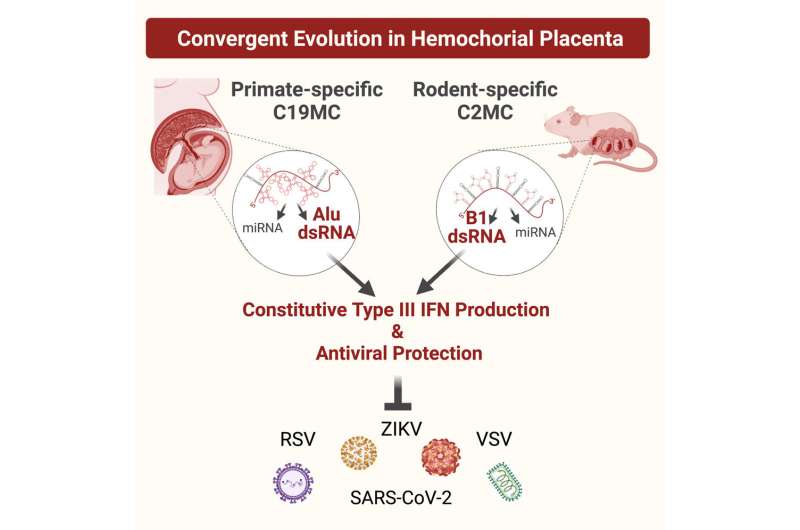
But the biological processes that protect a fetus from most viral infections are less clear. In the new paper, titled “SINE RNA of the imprinted miRNA clusters mediates constitutive type III interferon expression and antiviral protection in hemochorial placentas,” Dr. Totary-Jain and her team describe how a certain gene in the placenta is always armed for the battle.
“The placenta, in human and in mouse, is the first organ the fetus develops, and it is constantly exposed to maternal blood. This increases the chances of transmitting viral infections from the mother to the fetus. Therefore, the placenta has evolved robust defense mechanisms to prevent this transmission. We discovered a gene in the placenta that is expressed only from the paternal allele and produces a viral mimicry response. It tricks the placenta into thinking it’s infected and induces a constant state of antiviral defense,” Dr. Totary-Jain explained.
“So when we turned on this gene in other cells, we could protect the cells from several viruses. This is evolution’s way of protecting the baby. Without it, chances are you wouldn’t have made it into childbirth,: she added.
Ishani Wickramage, a Ph.D. candidate in Dr. Totary-Jain’s laboratory and a lead author of the stud, added, “This research fills the gap in our knowledge about how many viruses that may infect a pregnant mother, including SARS-CoV-2, only rarely affect the fetus.”‘
“Learning more about how the placenta shields the fetus from viruses also has important implications beyond childbirth,” said Dr. Charles Lockwood, MD, MHCM, one of the paper’s authors, who also is dean of the Morsani College of Medicine and executive vice president of USF Health.
“This is a novel placental mechanism that protects the developing fetus from transplacental transmission of most viruses,” Dr. Lockwood said. “This is the kind of knowledge that could lead to the development of new anti-viral medications to fight viruses that can be deadly for fetuses and newborn babies.”
Dr. Totary-Jain and a team of researchers at USF spent five years investigating this intriguing phenomenon in collaboration with Dr. Thomas Tuschl’s lab at Rockefeller University, whose team performed the sRNAseq and bioinformatic analysis, including researcher Klaas Max and Kemel Akat; and Drs. Kimiko Inoue and Atsuo Ogura from RIKEN and University of Tsukuba, Japan, who provided the mouse model that was used to show that the mouse placenta also developed the same mechanism to protect the fetus from viral infections.
Other USF Health members of the research team are: Jeffrey VanWye; John H. Lockhart; Ismet Hortu; Ezinne F. Mong; John Canfield; Hiran M. Lamabadu Warnakulasuriya Patabendige; Ozlem Guzeloglu-Kayisli; and Umit A. Kayisli.
More information:
Ishani Wickramage et al, SINE RNA of the imprinted miRNA clusters mediates constitutive type III interferon expression and antiviral protection in hemochorial placentas, Cell Host & Microbe (2023). DOI: 10.1016/j.chom.2023.05.018
Journal information:
Cell Host & Microbe
Source: Read Full Article