Lung mesenchymal cells, which are critical components of the lung’s unique structure, also play important roles in disease and recovery from injury, yet knowledge is limited about their biology or how they initiate diseases like pulmonary fibrosis. While experimental models have helped to identify some regulators of lung mesenchyme behavior, the understanding of how the lung mesenchyme is identified during human development is unknown.
To better understand these processes, researchers from Boston University Chobanian & Avedisian School of Medicine have developed an in-vitro induced pluripotent stem cell (iPSC)-based model system for the derivation and study of early lung-specific mesenchyme with potential benefit for comprehending basic mechanisms regulating tissue-specific mesenchymal fate decisions and future applications for regenerative medicine.
“Our study has implications for the study of lung diseases, such as pulmonary fibrosis and interstitial lung diseases that arise from dysfunction of the part of the lung known as mesenchyme. These diseases currently have very limited treatment options and we hope our model system will provide new tools to understand what goes wrong in these diseases and to screen for better drugs,” said corresponding author Darrell Kotton, MD, the David C. Seldin Professor of Medicine and director of the BU/Boston Medical Center Center for Regenerative Medicine (CReM).
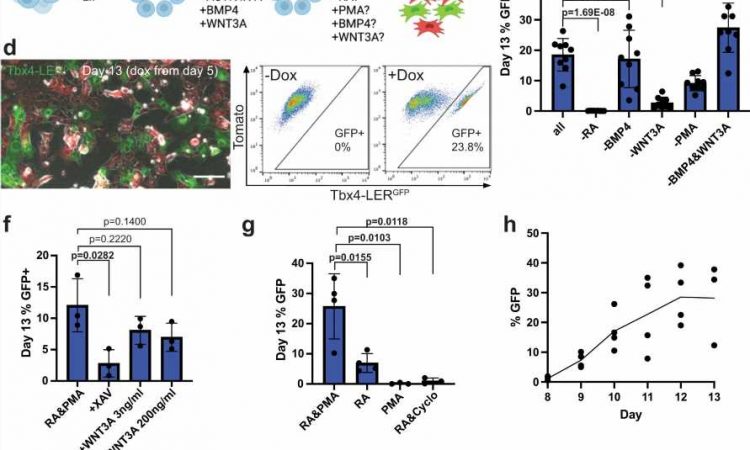
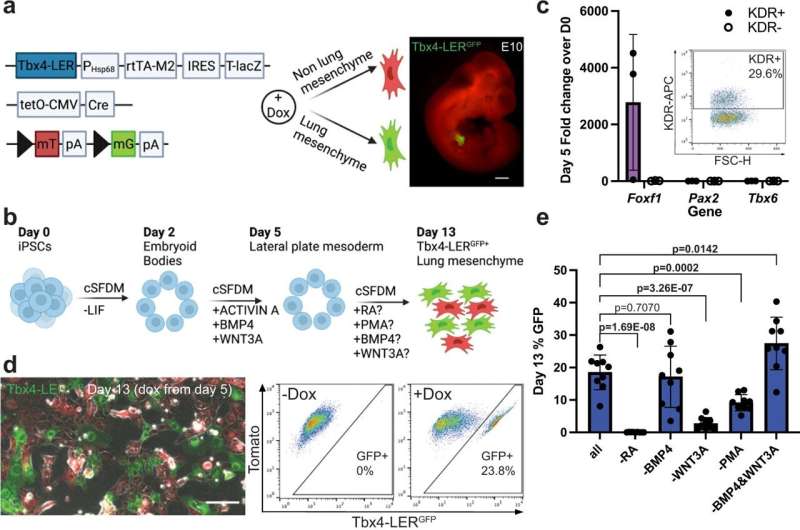
The researchers used an experimental model with an iPSC line carrying a lung mesenchyme-specific fluorescent reporter, meaning that cells that become lung mesenchymal were marked by green fluorescence. Using this model, they tested several growth factors and small molecules to stimulate pathways with known roles in lung development.
They found that stimulating the retinoic acid and hedgehog signaling pathways, both known to play essential roles in embryonic development, resulted in the maximum percentage of green fluorescent cells indicative of the potential presence of lung mesenchyme. They then isolated those cells and compared their gene expression profile to primary cells from embryonic lungs of the experimental model to determine how similar these cells are to primary lung mesenchymal cells.
Finally, they used their recombinant organoid system to test whether these cells can actually function as lung mesenchyme.
“An important role of the developing lung mesenchyme in the experimental model is their ability to interact with and signal to the neighboring epithelium. We found that our engineered cells can recapitulate some of those signaling interactions, suggesting that they have functional capacity,” explained first author Andrea Alber, Ph.D., a postdoctoral fellow in Kotton’s laboratory.
According to the researchers, the part of the study where the engineered lung mesenchymal cells are combined with lung epithelial cells in culture dishes (so called “recombinants”) is particularly exciting as this resulted in organoids, living cells assembled together in a 3D culture gel that helps scientists understand how cells are organized and communicate. “We are now working to apply these types of new organoid models to better understand pulmonary fibrosis,” added Alber.
These findings appear in the journal Nature Communications.
More information:
Andrea B. Alber et al, Directed differentiation of mouse pluripotent stem cells into functional lung-specific mesenchyme, Nature Communications (2023). DOI: 10.1038/s41467-023-39099-9
Journal information:
Nature Communications
Source: Read Full Article