It was previously believed that herpesviruses use certain body cells to replicate and other body cells to remain dormant, that is to remain inactive for a longer period of time. This dogma is now being questioned using the example of cytomegalovirus (CMV), a herpesvirus from the beta-herpesvirus subfamily, which can be fatal in immunocompromised transplant recipients.
In a new study, scientists from the Viral Immunology department at the Helmholtz Centre for Infection Research (HZI) in Braunschweig have discovered that certain connective tissue cells (fibroblasts) are not only used by CMV for replication, as previously assumed.
Apparently, CMV can also remain latent in the fibroblasts. The prevailing picture of an either/or—either the CMV uses a certain type of body cell for proliferation, or it remains in an inactive state there—is therefore no longer tenable. A second paradigm shift suggested by the study is the regulation of the CMV latency in cells: Apparently, the virus controls the use of fibroblasts as sites of latent or active infection not only via factors present in the cell, but also via an interaction with the immune system.
The results were published in Nature Communications.
As part of the new study, Dr. Katarzyna Sitnik, then working in the Viral Immunology department at the HZI, headed by Prof Luka Cicin-Sain, and her colleagues naturally infected mice with murine CMV (mCMV). This variant of the virus is used because human CMV (HCMV) is adapted to humans and cannot infect mice, while mCMV is the naturally occurring CMV infection in mice, causing a disease that is very similar to the one induced by HCMV in humans.
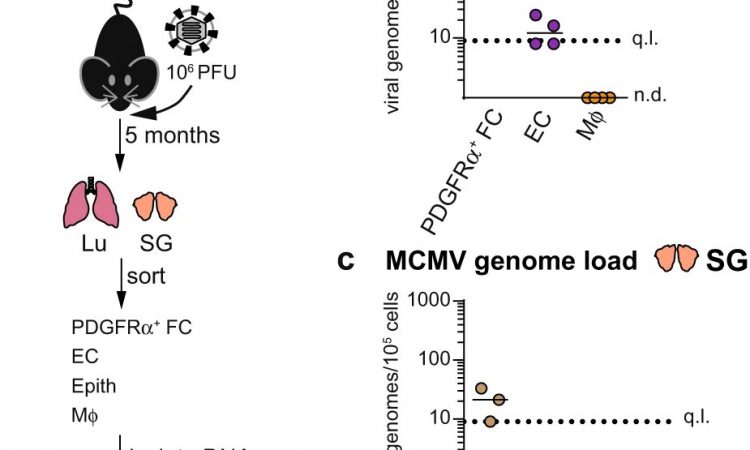
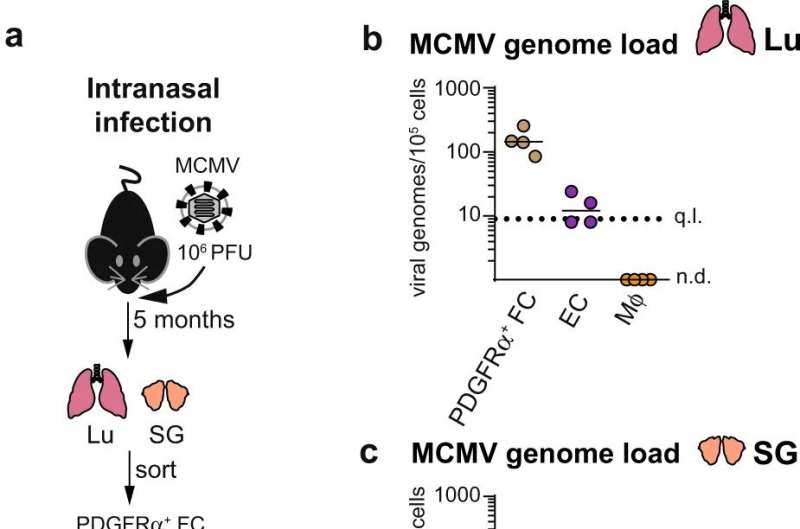
The scientists then carried out a systematic analysis of the cells that could carry the latent virus in experimentally infected mice.
The HZI team with colleagues from the University of Veterinary Medicine in Vienna and the medical faculty of the University of Rijeka, Croatia, were able to show that mCMV genomes are present in various cells of latently infected mice. However, a certain type of cells in connective tissue, so-called fibroblasts, stands out.
“We were surprised that fibroblasts of all things can serve as a residence for the latent CMV, because we use fibroblasts to grow this virus in tissue cultures,” says Prof Luka Cicin-Sain, head of the Viral Immunology department at the HZI.
The result contradicted the conventional wisdom that herpesviruses typically have a specialized cell type to maintain their dormancy and a different cell type in which to replicate. How could the same cells harbor latent and replicating CMV?
The authors first tested whether stromal cells support the growth of mCMV in natural environments in vivo. They confirmed the results previously shown in tissue culture: CMV also uses fibroblasts to grow in an organism.
“We naturally wondered how CMV latency is regulated. So we looked for immune reactions—and found what we were looking for,” says Katarzyna Sitnik, the first author of the study, who now works at the University of Veterinary Medicine, Vienna, where key immunological experiments were carried out.
The researchers found that while mCMV was able to replicate early after infection in mice lacking a particular molecule called STAT-1, it failed to maintain its genomes in a latent state. STAT-1 is critical for immunological signaling by interferons. “For us, this was the missing building block to explain how the virus manages to use the same cell type both for replication and for the long phases of latency,” says Sitnik.
So, this study represents a double paradigm shift: On the one hand, it shows that the same cells can be an important site of productive virus replication and latency. On the other hand, it shows that in the case of CMV, the latency is not regulated by intrinsic properties of a specific cell reservoir, but by the interaction of the virus with the immune system.
More information:
Katarzyna M. Sitnik et al, Fibroblasts are a site of murine cytomegalovirus lytic replication and Stat1-dependent latent persistence in vivo, Nature Communications (2023). DOI: 10.1038/s41467-023-38449-x
Journal information:
Nature Communications
Source: Read Full Article