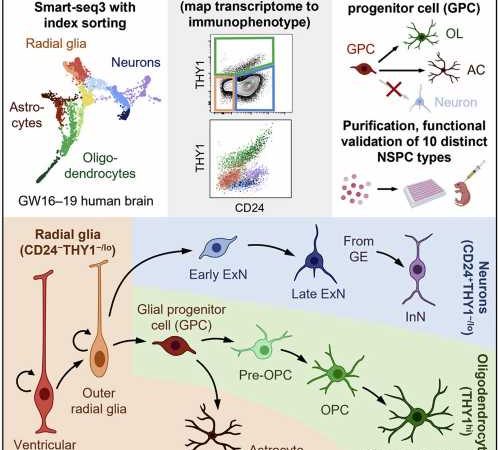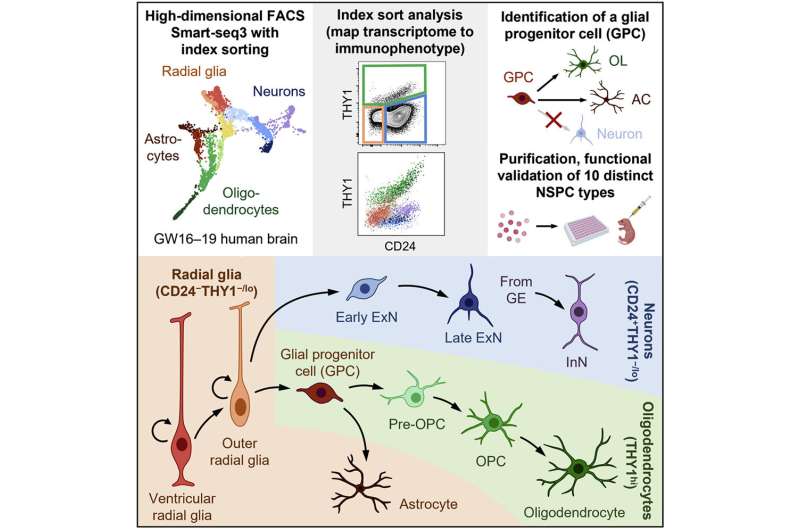
Researchers at Stanford University in California have devised a fluorescence-activated cell-sorting method for isolating distinct neural stem and progenitor cell types from human brain tissue. The markers used in the study are conserved across diverse brain regions. The technique should aid future research on neurodevelopment and accelerate the development of neuronal cell-transplantation-based therapeutic regimens to treat a host of neurological disorders.
In the research article, “Purification and characterization of human neural stem and progenitor cells,” published in the journal Cell, the Stanford team describes the combination of cutting-edge methods they used to develop a reliable isolation and identification scheme to capture the stem cells of interest.
The human brain is home to about 171 billion individual cells, with just over half (~86 billion) being neuronal cells. Those 86 billion neuronal cells are a diverse group, with hundreds of dedicated types and functions, but all originate from three neuronal lineages—neurons, oligodendrocytes and astrocytes. The three lineages all start from a pool of neural stem and progenitor radial glia cells that undergo rapid development during the second trimester of prenatal gestation. Understanding these radial glia cells, how they differentiate into the three lineages and how the three lineages differentiate into the diverse range of neuronal cells would be of enormous benefit to medical research.
Fluorescence-activated cell sorting was used to separate brain tissue cell types by cell surface immunophenotype from a suspension of single cells. The cells were indexed (fluorescence recorded) and isolated from each other. The cells were then submitted to single-cell RNA sequencing to capture their individual transcriptomes. By combining the surface-marker profile with indexing and transcriptome, the researchers now had a profile of each cell type that could be used for later identification. Researchers also measured the expression of 352 additional surface markers not used in the initial separation scheme but which could be used to better differentiate cells in the future.
Index-sort data allowed for each sequenced cell to be mapped back to its original immunophenotype and the researchers discovered that RNA and cell-surface protein expression did not always correlate. Some similar looking cells might have different functions. The strategy resulted in functionally similar populations of sorted cells allowing for the isolation of specific neural stem and progenitor cell type functions to be analyzed.
Ten neural stem and progenitor cell types were identified, and the researchers looked to characterize the behavior of the cells by transplanting them directly into the brains of neonatal immunodeficient mice. After six months, the cells had migrated and engrafted extensively throughout the brain and differentiated to give rise to all three major neural lineages. By observing how and where individual cell types propagated, the researchers could make some initial inferences about site-appropriate activities. Though the experiment was meant simply as a method viability test, researchers did identify and functionally characterize a distinct bipotent glial progenitor cell that had not been previously described.
The Stanford team’s successful proof of concept (with a discovery) that distinct cell types from the developing brain can be isolated based on surface markers could be readily adapted by other research scientists, as it operates on relatively standard research equipment. If the method used can be replicated for other stem cell types, there could be a surge in our understanding of specific functions, mechanisms and hierarchical roles that cells play in the brain or in other organs.
More information:
Daniel Dan Liu et al, Purification and characterization of human neural stem and progenitor cells, Cell (2023). DOI: 10.1016/j.cell.2023.02.017
Journal information:
Cell
Source: Read Full Article