The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection causes the coronavirus disease 2019 (COVID-19) with varying severity. Researchers have recently questioned whether disease progression can be predicted by using specific non-invasive methods during the early stages of infection.
In a recent medRxiv* study, researchers provide an interim analysis of the Prospective Validation of a Proteomic Urine Test for Early and Accurate Prognosis of the Critical Complications in Patients with SARS-CoV-2 Infection Study (CRIT-Cov-U) pilot study. To this end, the researchers described COV50, a urinary biomarker that can predict death and disease progression.
Study: Predictive performance and clinical application of COV50, a urinary proteomic biomarker in early COVID-19 infection: a cohort study. Image Credit: Kristini / Shutterstock.com
COV50
Urinary proteomic profiling (UPP) refers to the identification and generation of classifiers from the urine sample of a patient. In the case of SARS-CoV-2 infection, UPP is independent of the viral strain and may also inform treatment decisions in patients with COVID-19.
The CRIT-Cov-U discovered a novel UPP biomarker, COV50, consisting of 50 deregulated urinary peptides. COV50 can predict death and disease progression across the COVID-19 World Health Organization (WHO) scores over and beyond the risk factors and comorbidities.
The current study consolidates the findings of CRIT-Cov-U and proposes potential applications of the COV50 biomarker in clinical practice.
CRIT-Cov-U study
The prospective multicenter cohort study was conducted in eight European countries, including Austria, France, Germany, Greece, North Macedonia, Poland, Spain, and Sweden.
A total of 1,012 adults with COVID-19 confirmed by reverse-transcriptase polymerase chain reaction (RT-PCR) were enrolled in the study. According to the eight-point WHO clinical progression scale, all study participants were followed up for death and progression until recovery, hospital discharge, or death. Urine samples of the patients were analyzed and profiled using capillary electrophoresis coupled with mass spectrometry.
The researchers also computed hospitalization costs based on several factors, including the number of hospitalization days and care-facility costing rates. These hospitalization costs were standardized across the five countries.
Study findings
The study participants had WHO scores of 1-3 in 44% patients, 4-5 in 52.3% patients, and six in 3.8% patients. Of the 1,102 patients enrolled, 119 died, and 271 progressed, all of whom had an average age was 62.3 years.
Of the study participants, 44.2% were women, 55% had hypertension, 15.2% had heart failure, 25.4% had diabetes, and 10.5% had cancer. The odds associated with COV50 were 1.67-fold for death and 1.63-fold for disease progression after adjusting for sex, age, body mass index, comorbidities, and baseline WHO score.
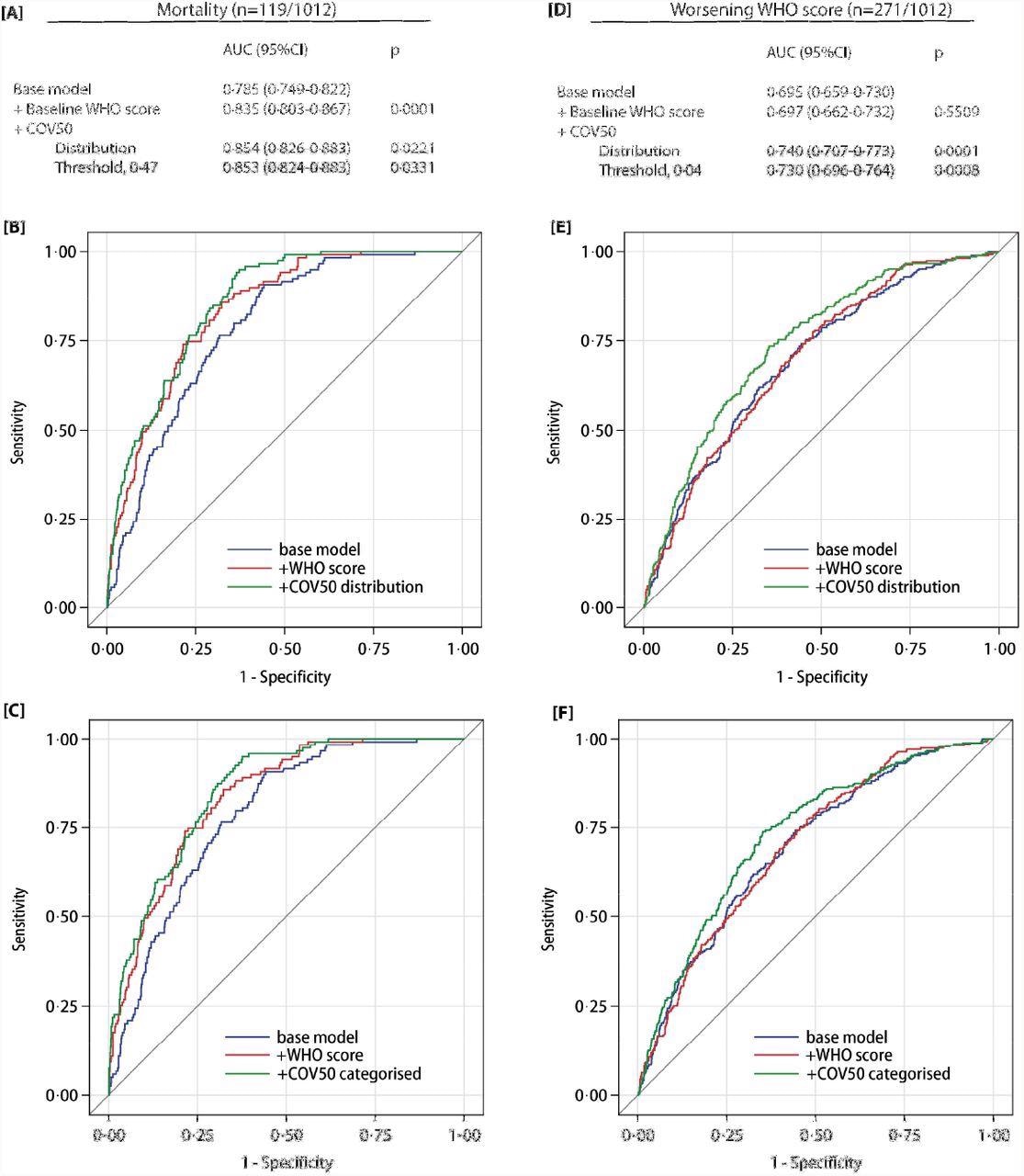
Performance of the COV50 urinary marker on top of other baseline risk factors in the full dataset for contrasting mortality vs survival (panels A-C) and for progression vs non-progression in the baseline WHO score during follow-up (panels D-F). The base model included sex, age, body mass index and the presence of comorbidities: hypertension, heart failure, diabetes or cancer. In subsequent steps, the baseline WHO score was added and next COV50 as a continuously distributed variable (panels B and E) or as a categorized variable based on an optimized threshold of 0·47 for mortality (panel C) or 0·04 for a worsening WHO score (panel F).
The prediction of the optimized COV50 0.47 threshold was 74.8% sensitive, 74.4% specific, and 74·4% accurate for mortality. Comparatively, the prediction of the 0.04 threshold was 67.5% sensitive, 67.3% specific, and 67.4% accurate for disease progression. Out of the 196 ambulatory patients, 194 did not reach the 0.04 threshold.
According to the current study, early intervention informed by the COV50 biomarker should reduce hospitalization days. As a result, the predicted hospitalization cost reductions will range from 1,208 million euros (M€) at low risk in patients with COV50 less than the 0.04 threshold. Comparatively, the cost reductions were estimated to be M€ 4,503 at high risk as defined as those with a COV50 threshold above 0.04 and over the age of 65 years.
Conclusions
The current study findings demonstrate that the COV50 biomarker is a novel urinary marker that accurately predicts COVID-19 outcomes. Furthermore, this is a non-invasive biomarker and is relatively inexpensive to complete compared to other hospitalization costs. In fact, COV50 is registered in Germany and is available for clinical testing and research in the European Union.
The urine sample of patients can be stored for five days at room temperature without affecting the UPP and can be stored at -20°C for future research. This allows remote testing of non-hospitalized patients with COVID-19.
COV50 can predict disease outcomes at the initial stage of SARS-CoV-2 infection. At this stage, disease progression is uncertain. A high-risk test outcome can help identify patients that require early administration of effective treatments.
Even in mild-to-moderate RT-PCR-confirmed SARS-CoV-2 infections, the 0.04 COV50 threshold justifies earlier drug treatment, which can ultimately reduce hospitalization days and costs. Aside from healthcare costs, cost-effectiveness can include non-monetary units like quality-adjusted life-years (QALYs), which were not addressed in this study.
Limitations
The current study is observational and does not provide the optimal strategy for COVID-19 treatment guided by COV50 risk profiling. The health-economic implications of the COV50 score and therapeutic decisions need to be studied. Furthermore, the current study only white European individuals; therefore, there is no available data on whether UPP is affected by ethnicity.
Although COV50 risk profiling can help make informed decisions regarding the therapeutic intervention, vaccination is still the most effective strategy in addressing the COVID-19 pandemic.
*Important notice
medRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
- Staessen, J. A., Wendt, R., Yu Y. Y., et al. (2022). Predictive performance and clinical application of COV50, a urinary proteomic biomarker in early COVID-19 infection: a cohort study. medRxiv. doi:10.1101/2022.01.20.22269599. https://www.medrxiv.org/content/10.1101/2022.01.20.22269599v1.
Posted in: Medical Research News | Medical Condition News | Disease/Infection News | Healthcare News
Tags: Biomarker, Body Mass Index, Cancer, Clinical Testing, Coronavirus, Coronavirus Disease COVID-19, Diabetes, Electrophoresis, Healthcare, Heart, Heart Failure, Hospital, Mass Spectrometry, Mortality, Pandemic, Peptides, Polymerase, Polymerase Chain Reaction, Research, Respiratory, SARS, SARS-CoV-2, Severe Acute Respiratory, Severe Acute Respiratory Syndrome, Spectrometry, Syndrome, Urine Analysis, Urine Test

Written by
Dr. Shital Sarah Ahaley
Dr. Shital Sarah Ahaley is a medical writer. She completed her Bachelor's and Master's degree in Microbiology at the University of Pune. She then completed her Ph.D. at the Indian Institute of Science, Bengaluru where she studied muscle development and muscle diseases. After her Ph.D., she worked at the Indian Institute of Science, Education, and Research, Pune as a post-doctoral fellow. She then acquired and executed an independent grant from the DBT-Wellcome Trust India Alliance as an Early Career Fellow. Her work focused on RNA binding proteins and Hedgehog signaling.
Source: Read Full Article
