WHO safety experts prepared to meet Tuesday over the AstraZeneca coronavirus vaccine whose rollout has been halted in several European countries over blood clot fears, imperilling the pandemic fight as infection rates surge.
The three largest EU nations—Germany, Italy and France—joined others in suspending the shot Monday, dealing a blow to the global immunisation campaign against a disease that has killed more than 2.6 million people.
The World Health Organization, AstraZeneca, and the European Medicines Agency have insisted the shot is safe, and that there is no link between the vaccine and reported blood clots.
“We do not want people to panic and we would, for the time being, recommend that countries continue vaccinating with AstraZeneca,” WHO chief scientist Soumya Swaminathan said Monday.
“So far, we do not find an association between these events and the vaccine.”
WHO and EMA experts on Tuesday will separately discuss data from AstraZeneca vaccinations, and the European regulator will hold an extraordinary meeting two days later to decide on any further action.
AstraZeneca’s shot, among the cheapest available, was billed as the vaccine of choice for poorer nations and the clot reports have had an impact beyond Europe.
Indonesia delayed its AstraZeneca rollout on Monday, and Venezuela announced it would not authorise the jab over fears of “complications”.
The vaccine was developed by AstraZeneca and the University of Oxford in Britain, where more than 11 million doses have been administered apparently without any major problems.
Prime Minister Justin Trudeau on Monday also urged Canadians to get the AstraZeneca shot after reports of hesitancy based on the suspensions in Europe.
‘Critical situation’
The AstraZeneca crisis comes as a number of countries battle worrying surges in coronavirus infections, a grim reminder that the battle against Covid-19 is far from over.
Norway’s capital Oslo announced tougher measures to stop the spread of the virus, including closing secondary schools, as it reported a record number of cases last week.
“These will be the most intrusive measures taken by Oslo during the pandemic,” said mayor Raymond Johansen.
“It’s tough, it’s difficult but it’s necessary”.

And a fresh spike pushed the main Covid-19 hospital in Bosnia to the edge, forcing it to declare a state of emergency.
“The staff is exhausted,” hospital director Sebija Izetbegovic wrote on Facebook.
“We will continue to do what is possible to save lives, but the situation is really critical. More and more of our employees are sick.”
Most of Italy re-entered lockdown on Monday, with schools, restaurants, shops and museums closed, while intensive care doctors in Germany issued an urgent appeal for new restrictions to avoid a third wave in the country.
Brazil’s President Jair Bolsonaro—widely panned for his coronavirus scepticism—appointed a new health minister Monday as the South American nation reeled from another deadly surge in infections and deaths.
Vaccine options
The pandemic spurred unprecedented efforts to develop vaccines, with a number of successful options now available.
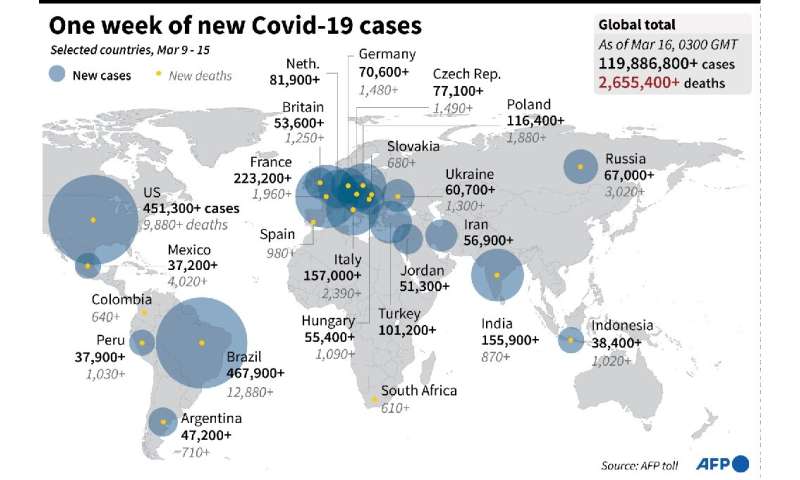
Rollouts have been hampered, however, by export controls, bitter diplomatic spats and production issues—in addition to the AstraZeneca suspension.
But a new agreement for Germany’s IDT Biologika to help produce the single-shot Johnson & Johnson vaccine would offer Europe greater certainty, Germany’s economy minister said Monday.
And the developers of Russia’s successful Sputnik V vaccine said Monday they had reached production agreements in key European countries.
China, where the virus first emerged in late 2019, has also developed Covid-19 vaccines and begun exporting them across the world—including to Europe, the Middle East and South America.

On Monday, it pledged 300,000 doses to protect United Nations peacekeepers, adding to the 200,000 doses already pledged by India. Neither country specified which type of vaccines would be donated.
China has largely brought its outbreak under control, but maintains strict travel restrictions to avoid importing cases.
Source: Read Full Article
